Abstract
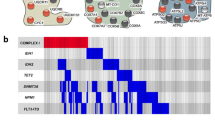
To study the prevalence and prognostic importance of mutations in NADH dehydrogenase subunit 4 (ND4), a mitochondrial encoded transmembrane component of the electron transport chain respiratory Complex I, 452 AML patients were examined for ND4 mutations by direct sequencing. The prognostic impact of ND4 mutations was evaluated in the context of other clinical prognostic markers and genetic risk factors. In all, 29 of 452 patients (6.4%) had either somatic (n=12) or germline (n=17) ND4 mutations predicted to affect translation. Somatic mutations were more likely to be heteroplasmic (P<0.001), to occur in predicted transmembrane domains (P<0.001) and were predicted to have damaging effects upon translation (P<0.001). Patients with somatically acquired ND4 mutations had significantly longer relapse-free survival (P=0.017) and overall survival (OS) (P=0.021) than ND4wildtype patients. Multivariate analysis also demonstrated a tendency for increased survival in patients with somatic ND4 mutations (RFS: hazard ratio (HR) 0.25, confidence interval (CI) 0.06–1.01, P=0.052; OS: HR 0.29, CI 0.74–1.20, P=0.089). Somatic ND4mutated patients had a higher prevalence of concomitant DNMT3A mutations (P=0.023) and a higher percentage of the NPM1/FLT3-ITD low-risk genotype (P=0.021). Germline affected cases showed higher BAALC (P=0.036) and MLL5 (P=0.051) expression levels. Further studies are warranted to validate the favorable prognostic influence of acquired ND4 mutations in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Mrozek K, Heerema NA, Bloomfield CD . Cytogenetics in acute leukemia. Blood Rev 2004; 18: 115–136.
Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008; 358: 1909–1918.
Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 2008; 456: 66–72.
Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010; 363: 2424–2433.
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009; 361: 1058–1066.
Gogvadze V, Orrenius S, Zhivotovsky B . Mitochondria as targets for cancer chemotherapy. Semin Cancer Biol 2009; 19: 57–66.
Brandon M, Baldi P, Wallace DC . Mitochondrial mutations in cancer. Oncogene 2006; 25: 4647–4662.
DiMauro S, Schon EA . Mitochondrial respiratory-chain diseases. N Engl J Med 2003; 348: 2656–2668.
Ishikawa K, Hayashi J . A novel function of mtDNA: its involvement in metastasis. Ann NY Acad Sci 2010; 1201: 40–43.
Lee HC, Chang CM, Chi CW . Somatic mutations of mitochondrial DNA in aging and cancer progression. Ageing Res Rev 2010; 9 (Suppl 1): S47–S58.
Piccoli C, Ripoli M, Scrima R, Stanziale P, Di Ianni M, Moretti L et al. MtDNA mutation associated with mitochondrial dysfunction in megakaryoblastic leukaemic cells. Leukemia 2008; 22: 1938–1941.
Manea EM, Leverger G, Bellmann F, Stanescu PA, Mircea A, Lebre AS et al. Pearson syndrome in the neonatal period: two case reports and review of the literature. J Pediatr Hematol Oncol 2009; 31: 947–951.
He L, Luo L, Proctor SJ, Middleton PG, Blakely EL, Taylor RW et al. Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia 2003; 17: 2487–2491.
Yao YG, Ogasawara Y, Kajigaya S, Molldrem JJ, Falcao RP, Pintao MC et al. Mitochondrial DNA sequence variation in single cells from leukemia patients. Blood 2007; 109: 756–762.
Torres-Bacete J, Nakamaru-Ogiso E, Matsuno-Yagi A, Yagi T . Characterization of the NuoM (ND4) subunit in Escherichia coli NDH-1: conserved charged residues essential for energy-coupled activities. J Biol Chem 2007; 282: 36914–36922.
Groschel S, Lugthart S, Schlenk RF, Valk PJ, Eiwen K, Goudswaard C et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol 2010; 28: 2101–2107.
Heil G, Krauter J, Raghavachar A, Bergmann L, Hoelzer D, Fiedler W et al. Risk-adapted induction and consolidation therapy in adults with de novo AML aged </= 60 years: results of a prospective multicenter trial. Ann Hematol 2004; 83: 336–344.
Mitelman F . ISCN (1995): An International System for Human Cytogenetic Nomenclature. S. Karger: Basel, Switzerland, 1995.
Wagner K, Damm F, Gohring G, Gorlich K, Heuser M, Schafer I et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol 2010; 28: 2356–2364.
Thol F, Damm F, Wagner K, Gohring G, Schlegelberger B, Hoelzer D et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood 2010; 116: 614–616.
Thol F, Damm F, Ludeking A, Winschel C, Wagner K, Morgan M et al. Incidence and Prognostic Influence of DNMT3A Mutations in Acute Myeloid Leukemia. J Clin Oncol 2011; 29: 2889–2896.
Frohling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol 2004; 22: 624–633.
Damm F, Heuser M, Morgan M, Yun H, Grosshennig A, Gohring G et al. Single nucleotide polymorphism in the mutational hotspot of WT1 predicts a favorable outcome in patients with cytogenetically normal acute myeloid leukemia. J Clin Oncol 2010; 28: 578–585.
Baldus CD, Martus P, Burmeister T, Schwartz S, Gokbuget N, Bloomfield CD et al. Low ERG and BAALC expression identifies a new subgroup of adult acute T-lymphoblastic leukemia with a highly favorable outcome. J Clin Oncol 2007; 25: 3739–3745.
Marcucci G, Maharry K, Whitman SP, Vukosavljevic T, Paschka P, Langer C et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol 2007; 25: 3337–3343.
Heuser M, Beutel G, Krauter J, Dohner K, von Neuhoff N, Schlegelberger B et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood 2006; 108: 3898–3905.
Langer C, Marcucci G, Holland KB, Radmacher MD, Maharry K, Paschka P et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol 2009; 27: 3198–3204.
Damm F, Oberacker T, Thol F, Surdziel E, Wagner K, Chaturvedi A et al. Prognostic importance of histone methyltransferase Mixed Lineage Leukemia 5 (MLL5) expression in acute myeloid leukemia. J Clin Oncol 2011; 29: 682–689.
Heuser M, Argiropoulos B, Kuchenbauer F, Yung E, Piper J, Fung S et al. MN1 overexpression induces acute myeloid leukemia in mice and predicts ATRA resistance in patients with AML. Blood 2007; 110: 1639–1647.
Damm F, Heuser M, Morgan M, Wagner K, Gorlich K, Grosshennig A et al. Integrative prognostic risk score in acute myeloid leukemia with normal karyotype. Blood 2011; 117: 4561–4568.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Korn EL . Censoring distributions as a measure of follow-up in survival analysis. Stat Med 1986; 5: 255–260.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–2333.
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010; 17: 225–234.
Prensner JR, Chinnaiyan AM . Metabolism unhinged: IDH mutations in cancer. Nat Med 2011; 17: 291–293.
Lerner F, Niere M, Ludwig A, Ziegler M . Structural and functional characterization of human NAD kinase. Biochem Biophys Res Commun 2001; 288: 69–74.
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 2009; 324: 261–265.
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011; 19: 17–30.
Mayr JA, Meierhofer D, Zimmermann F, Feichtinger R, Kogler C, Ratschek M et al. Loss of complex I due to mitochondrial DNA mutations in renal oncocytoma. Clin Cancer Res 2008; 14: 2270–2275.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Jeronimo C, Nomoto S, Caballero OL, Usadel H, Henrique R, Varzim G et al. Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene 2001; 20: 5195–5198.
Acknowledgements
We thank all patients and physicians for provision of study material; Kerstin Görlich, Elvira Lux, Sylvia Horter, Diana Dudacy, Irina Schäfer, Susanne Wolf and Uwe Borchert for their support in sample and data acquisition; Dr Sarvari Velaga for helping in the preparation of Figure 2; Dr Thomas Winkler and Dr Olivier Bernard for critically reading the manuscript. We acknowledge the assistance of the Cell-Sorting-Core-Facility of the Hannover Medical School supported in part by Braukmann-Wittenberg-Herz-Stiftung and Deutsche Forschungsgemeinschaft. This study was supported by the Hannelore-Munke Fellowship, grants HILF 2010 and no. 109686 by the Deutsche Krebshilfe awarded to FD; grant no. M 47.1 from the HW & J Hector Stiftung; and grants no. 01GI0378 (Kompetenznetz ‘Akute und chronische Leukämien’) and 01KG0605 from the Bundesministerium für Bildung und Forschung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Damm, F., Bunke, T., Thol, F. et al. Prognostic implications and molecular associations of NADH dehydrogenase subunit 4 (ND4) mutations in acute myeloid leukemia. Leukemia 26, 289–295 (2012). https://doi.org/10.1038/leu.2011.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.200
Keywords
This article is cited by
-
Understanding the molecular evolution of tiger diversity through DNA barcoding marker ND4 and NADH dehydrogenase complex using computational biology
Genes & Genomics (2021)
-
Genetic and molecular biology of breast cancer among Iranian patients
Journal of Translational Medicine (2019)
-
Next-generation sequencing identifies novel mitochondrial variants in pituitary adenomas
Journal of Endocrinological Investigation (2019)
-
ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome
Leukemia (2013)
-
Myeloid malignancies: mutations, models and management
BMC Cancer (2012)