Abstract
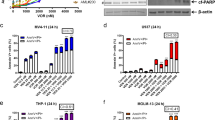
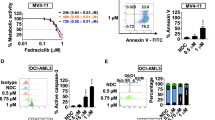
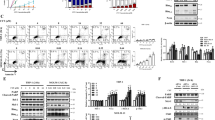
Acute myeloid leukemia (AML) cells express the cell surface antigen CD33 that, upon ligation with a monoclonal antibody (mAb), is a downregulator of cell growth in a Syk-dependent manner. An anti-CD33 mAb coupled to a toxin, gemtuzumab ozogamicin (GO), is used for the treatment of AML (Mylotarg). Therefore, we investigated whether the response of AML cells to GO treatment also depends on Syk expression. Forty primary AML samples (25 Syk-positive and 15 Syk-negative) were tested for their response to the anti-proliferative effects of GO and unmodified anti-CD33 mAb. A correlation between Syk expression and the response of leukemia cells to GO and anti-CD33 mAb was found. ‘Blocking’ of Syk by small interfering RNA resulted in unresponsiveness of AML cells to both GO and anti-CD33 mAb-mediated cytotoxicity. Syk upregulation by the de-methylating agent 5-azacytidine (5-aza) induced re-expression of Syk in some cases, resulting in enhanced GO and anti-CD33-mediated inhibition of leukemia cell growth. Thus, the cytotoxicity of both GO and anti-CD33 in primary AML samples was associated with Syk expression. 5-Aza restored Syk and increased the sensitivity of originally Syk-negative, non-responsive cells to CD33 ligation to levels of Syk-positive cells. These data have clinical significance for predicting response to GO and designing clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF . A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leukemia Res 1984; 8: 521–527.
Sievers EL, Appelbaum FR, Spielberger RT . Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamycin immunoconjugate. Blood 1999; 93: 3678–3684.
Freeman SD, Kelm S, Barber EK, Crocker PR . Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 1995; 85: 2005–2012.
Ulyanova T, Blasioli J, Woodford-Thomas TA, Thomas ML . The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur J Immunol 1999; 29: 3440–3449.
Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD . The myeloid-specific scialic acid-binding receptor, CD33, associates with the protein tyrosine phosphatases, SHP-1 and SHP-2. J Biol Chem 1999; 274: 11505–11512.
Paul SP, Taylor LS, Sansbury EK, McVicar DW . Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 2000; 96: 483–490.
Vitale C, Romagnani C, Falco M, Ponte M, Mingari MC . Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc Natl Acad Sci USA 1999; 96: 15091–15096.
Vitale C, Romagnani C, Puccetti A, Olive D, Mingari MC . Surface expression and function of p75/AIRM-1 or CD33 in acute myeloid leukemias: engagement of CD33 induces apoptosis of leukemic cells. Proc Natl Acad Sci USA 2001; 98: 5764–5769.
Mingari MC, Vitale C, Romagnani C, Falco M, Moretta L . p75/AIRM1 and CD33, two sialoadhesin receptors that regulate the proliferation or the survival of normal and leukemic myeloid cells. Immunol Rev 2001; 181: 260–268.
Bae J, Martinson JA, Klingemann HG . Heteroclitic CD33 peptide with enhanced anti-acute myeloid leukemic immunogenicity. Clin Cancer Res 2004; 10: 7043–7052.
Sievers EL . Targeted therapy of acute myeloid leukemia with monoclonal antibodies and immunoconjugates. Cancer Chemother Pharmacol 2000; 46: 18–22.
Qin S, Yamamura H . Up-regulation of Syk activity during HL60 cell differentiation into granulocyte but not into monocyte/macrophage-lineage. Biochem Biophys Res Commun 1997; 236: 697–701.
Tsubokawa M, Tohyama Y, Tohyama K, Asahi M, Inazu T, Nakamura H et al. Interleukin-3 activates Syk in a human myeloblastic leukemia cell line, AML-193. Eur J Biochem 1997; 249: 792–796.
Raeder EM, Mansfield PJ, Hinkovska-Galcheva V, Shayman JA, Boxer LA . Syk activation initiates downstream signaling events during human polymorphonuclear leukocyte phagocytosis. J Immunol 1999; 163: 6785–6793.
Cambien B, Pomeranz M, Millet MA, Rossi B, Schmid-Alliana A . Signal transduction involved in MCP-1-mediated monocytic trans-endothelian migration. Blood 2001; 97: 359–366.
Nakashima K, Kokubo T, Shichijo M, Li YF, Yura T, Yamamoto N . A novel Syk kinase-selective inhibitor blocks antigen presentation of immune complexes in dendritic cells. Eur J Pharmacol 2004; 505: 223–228.
Shen L, Lang ML, Wade WF . The ins and outs of getting in: structures and signals that enhance BCR or Fc receptor-mediated antigen presentation. Immunopharmacology 2000; 49: 227–240.
Dustin LB, Plas DR, Wong J, Hu YT, Soto C, Chan AC et al. Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase –1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol 1999; 162: 2717–2724.
Kraft S, Katoh N, Novak N, Koch S, Bieber T . Unexpected functions of FcepsilonRI on antigen-presenting cells. Int Arch Allergy Immunol 2001; 124: 35–37.
Hamawy MM, Fischler C, Zhang J, Siraganian RP . Fc epsilon RI aggregation induces tyrosine phosphorylation of a novel 72 kDa protein downstream of Syk. Biochem Biophys Res Commun 1997; 239: 670–675.
Xu R, Seger R, Pecht I . Cutting edge: extracellular signal-regulated kinase activates syk: a new potential feedback regulation of Fc epsilon receptor signaling. J Immunol 1999; 163: 1110–1114.
Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL . Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol 2001; 166: 1507–1516.
Ebel C, Schmidt RE, Hundt M . Signal transduction via both human low-affinity IgG Fc receptors, Fc gamma RIIa and Fc gamma RIIIb, depends on the activity of different families of intracellular kinases. Immunobiology 2001; 203: 616–628.
Law CL, Sidorenko SP, Chandran KA, Zhao Z, Shen SH, Fischer EH et al. CD22 associates with protein tyrosine phosphatase 1C, Syk and phospholipase C-gamma 1 upon B cell activation. J Exp Med 1996; 183: 547–560.
Tuscano JM, Engel P, Tedder TF, Agarval A, Kehrl JH . Involvement of p72syk kinase, p53/56lyn kinase and phosphatidyl inositol-3 kinase in signal transduction via the human B lymphocyte antigen CD22. Eur J Immunol 1996; 26: 1246–1252.
Yohannan J, Wienands J, Coggeshall KM, Justement LB . Analysis of tyrosine phosphorylation-dependent interactions between stimulatory effector proteins and the B cell co-receptor CD22. J Biol Chem 1999; 274: 18769–18776.
Otipoby KL, Draves KE, Clark EA . CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem 2001; 276: 44315–44322.
Balaian L, Ball ED . Direct effect of bispecific anti-CD33 × anti-CD64 antibody on proliferation and signaling in myeloid cells. Leukemia Res 2001; 25: 1115–1125.
Balaian L, Zhong RK, Ball ED . The inhibitory effect of anti-CD33 monoclonal antibodies on AML cells growth correlates with Syk and/or ZAP-70 expression. Exp Hematol 2003; 5: 363–371.
Goodman PA, Wood CM, Vassilev A, Mao C, Uckun FM . Spleen tyrosine kinase (Syk) deficiency in childhood pro-B cell acute lymphoblastic leukemia. Oncogene 2001; 20: 3969–3978.
Goodman PA, Burkhardt N, Juran B, Tibbles HE, Uckun FM . Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene 2003; 22: 2504–2514.
Ushmorov A, Leithauser F, Sakk O, Weinhausel A, Popov S, Moller P et al. Epigenetic process play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood 2006; 107: 2493–2500.
Coopman PJ, Mueller SC . The Syk tyrosine kinase: a new negative regulator in tumor growth and progression. Cancer Lett 2006; 241: 159–173.
Stewart ZA, Pieterpol JA . Syk: a new player in the field of breast cancer. Breast Cancer Res 2001; 3: 5–7.
Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 2000; 406: 742–747.
Yuan Y, Mendez R, Sahin A, Dai JL . Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res 2001; 61: 5558–5561.
Yuan Y, Liu H, Sahin A, Le Dai J . Reactivation of syk expression by inhibition of DNA methylation suppresses breast cancer cell invasiveness. Int J cancer 2005; 113: 654–659.
Jabbour EJ, Giles EJ . New agents in myelodysplastic syndromes. Curr Hematol Rep 2005; 4: 191–199.
Fenaux P . Inhibitors of DNA methylation: beyond myelodysplastic syndromes. Natl Clin Pract Oncol 2005; 2: 836–844.
Claus L, Almstedt M, Lubbert M . Epigenetic treatment of hematopoietic malignancies: in vivo targets of demethylating agents. Semin Oncol 2005; 32: 511–520.
Balaian L, Ball ED . Anti-CD33 monoclonal antibodies enhance the cytotoxic effects of cytosine arabinoside and idarubicin on acute myeloid leukemia (AML) cells through similarities in their signaling pathways. Exp Hematol 2005; 33: 199–211.
Esteller M . DNA methylation and cancer therapy: new developments and expectations. Curr Opin Oncol 2005; 17: 55–60.
Acknowledgements
Larisa Balain designed and performed all of the experiments and prepared the paper. Edward D Ball co-designed the experiments and co-wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaian, L., Ball, E. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia 20, 2093–2101 (2006). https://doi.org/10.1038/sj.leu.2404437
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404437
Keywords
This article is cited by
-
A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome
Leukemia (2016)
-
The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells
Leukemia (2012)
-
Therapy with azanucleosides for myelodysplastic syndromes
Nature Reviews Clinical Oncology (2010)
-
Analysis of factors that affect in vitro chemosensitivity of leukaemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukaemia
Leukemia (2010)