Abstract
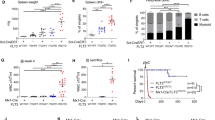
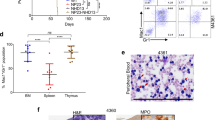
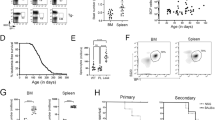
Rearrangement of the mixed lineage leukaemia (MLL) gene with extra eleven nineteen (EEN) was previously identified in an infant with acute myeloid leukaemia. Using homologous recombination, we have created a mouse equivalent of the human MLL-EEN allele and showed that when MllEen/+ embryonic stem (ES) cells were induced to differentiate in vitro into haemopoietic cells, there was increased proliferation of myeloid progenitors with self-renewal property. We also generated MllEen/+ chimeric mice, which developed leukaemia displaying enlarged livers, spleens, thymuses and lymph nodes owing to infiltration of MllEen/+-expressing leukemic cells. Immunophenotyping of cells from enlarged organs and bone marrow (BM) of the MllEen/+ chimeras revealed an accumulation of Mac-1+/Gr-1− immature myeloid cells and a reduction in normal B- and T-cell populations. We observed differential regulation of Hox genes between myeloid cells derived from MllEen/+ ES cells and mouse BM leukemic cells which suggested different waves of Hox expression may be activated by MLL fusion proteins for initiation (in ES cells) and maintenance (in leukemic cells) of the disease. We believe studies of MLL fusion proteins in ES cells combined with in vivo animal models offer new approaches to the dissection of molecular events in multistep pathogenesis of leukaemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa III R, Patel Y, Harden A et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA 1991; 88: 10735–10739.
Djabali M, Selleri L, Parry P, Bower M, Young BD, Evans GA . A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet 1992; 2: 113–118.
Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ . Altered Hox expression and segmental identity in Mll-mutant mice. Nature 1995; 378: 505–508.
Hess JL, Yu BD, Li B, Hanson R, Korsmeyer SJ . Defects in yolk sac hematopoiesis in Mll-null embryos. Blood 1997; 90: 1799–1806.
Look AT . Oncogenic transcription factors in the human acute leukemias. Science 1997; 278: 1059–1064.
Super HJ, McCabe NR, Thirman MJ, Larson RA, Le Beau MM, Pedersen-Bjergaard J et al. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood 1993; 82: 3705–3711.
Domer PH, Head DR, Renganathan N, Raimondi SC, Yang E, Atlas M . Molecular analysis of 13 cases of MLL/11q23 secondary acute leukemia and identification of topoisomerase II consensus-binding sequences near the chromosomal breakpoint of a secondary leukemia with the t(4;11). Leukemia 1995; 9: 1305–1312.
Broeker PL, Super HG, Thirman MJ, Pomykala H, Yonebayashi Y, Tanabe S et al. Distribution of 11q23 breakpoints within the MLL breakpoint cluster region in de novo acute leukemia and in treatment-related acute myeloid leukemia: correlation with scaffold attachment regions and topoisomerase II consensus binding sites. Blood 1996; 87: 1912–1922.
Chen CS, Sorensen PH, Domer PH, Reaman GH, Korsmeyer SJ, Heerema NA et al. Molecular rearrangements on chromosome 11q23 predominate in infant acute lymphoblastic leukemia and are associated with specific biologic variables and poor outcome. Blood 1993; 81: 2386–2393.
Pui CH, Behm FG, Downing JR, Hancock ML, Shurtleff SA, Ribeiro RC et al. 11q23/MLL rearrangement confers a poor prognosis in infants with acute lymphoblastic leukemia. J Clin Oncol 1994; 12: 909–915.
Ayton PM, Cleary ML . Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 2001; 20: 5695–5707.
Daser A, Rabbitts TH . The versatile mixed lineage leukaemia gene MLL and its many associations in leukaemogenesis. Semin Cancer Biol 2005; 15: 175–188.
Lavau C, Szilvassy SJ, Slany R, Cleary ML . Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J 1997; 16: 4226–4237.
Lavau C, Du C, Thirman M, Zeleznik-Le N . Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J 2000; 19: 4655–4664.
Lavau C, Luo RT, Du C, Thirman MJ . Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci USA 2000; 97: 10984–10989.
DiMartino JF, Miller T, Ayton PM, Landewe T, Hess JL, Cleary ML et al. A carboxy-terminal domain of ELL is required and sufficient for immortalization of myeloid progenitors by MLL-ELL. Blood 2000; 96: 3887–3893.
DiMartino JF, Ayton PM, Chen EH, Naftzger CC, Young BD, Cleary ML . The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10. Blood 2002; 99: 3780–3785.
So CW, Cleary ML . MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function. Mol Cell Biol 2002; 22: 6542–6552.
So CW, Cleary ML . Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood 2003; 101: 633–639.
So CW, Lin M, Ayton PM, Chen EH, Cleary ML . Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell 2003; 4: 99–110.
So CW, Karsunky H, Passegue E, Cozzio A, Weissman IL, Cleary ML . MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell 2003; 3: 161–171.
Ono R, Nakajima H, Ozaki K, Kumagai H, Kawashima T, Taki T et al. Dimerization of MLL fusion proteins and FLT3 activation syngergize to induce multiple-lineage leukemogenesis. J Clin Invest 2005; 115: 919–929.
Corral J, Lavenir I, Impey H, Warren AJ, Forster A, Larson TA et al. An Mll-AF9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell 1996; 85: 853–861.
Collins EC, Pannell R, Simpson EM, Forster A, Rabbitts TH . Inter- chromosomal recombination of Mll and Af9 genes mediated by cre-loxP in mouse development. EMBO Rep 2000; 1: 127–132.
Forster A, Pannell R, Drynan LF, McCormack M, Collins EC, Daser A et al. Engineering de novo reciprocal chromosomal translocations associated with Mll to replicate primary events of human cancer. Cancer Cell 2003; 3: 449–458.
Wang J, Iwasaki H, Krivtsov A, Febbo PG, Thorner AR, Ernst P et al. Conditional MLL-CBP targets GMP and models therapy-related myeloproliferative disease. EMBO J 2005; 24: 368–381.
Chen W, Li Q, Hudson WA, Kumur A, Kirchhof N, Kersey JH . A murine Mll-AF4 knockin model results in lymphoid and myeloid deregulation and hematological malignancy. Blood 2006; 108: 669–677.
Metzler M, Forster A, Pannell R, Arends MJ, Daser A, Lobato MN et al. A conditional model of MLL-AF4 B-cell tumourigenesis using invertor technology. Oncogene 2006; 25: 3093–3103.
Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet 2002; 30: 41–47.
Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood 2004; 104: 3679–3687.
Kohlmann A, Schoch C, Dugas M, Schnittger S, Hiddemann W, Kern W et al. New insights into MLL gene rearranged acute leukemias using gene expression profiling: shared pathways, lineage commitment, and partner genes. Leukemia 2005; 19: 953–964.
Ayton PM, Cleary ML . Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev 2003; 17: 2298–2307.
Kumar AR, Hudson WA, Chen W, Nishiuchi R, Yao Q, Kersey JH . Hoxa9 influences the phenotype but not the incidence of Mll-AF9 fusion gene leukemia. Blood 2004; 103: 1823–1828.
Zeisig BB, Milne T, Garcia-Cuellar MP, Schreiner S, Martin ME, Fuchs U et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol Cell Biol 2004; 24: 617–628.
So CW, Karsunky H, Wong P, Weissman IL, Cleary ML . Leukemic transformation of hematopoietic progenitors by MLL-GAS7 in the absence of Hoxa7 or Hoxa9. Blood 2004; 103: 3192–3199.
Johnson JJ, Chen W, Hudson W, Yao Q, Taylor M, Rabbitts TH et al. Prenatal and postnatal myeloid cells demonstrate stepwise progression in the pathogenesis of MLL fusion gene leukemia. Blood 2003; 101: 3229–3235.
So CW, Caldas C, Liu MM, Chen SJ, Huang QH, Gu LJ et al. EEN encodes for a member of a new family of proteins containing an Src homology 3 domain and is the third gene located on chromosome 19p13 that fuses to MLL in human leukemia. Proc Natl Acad Sci USA 1997; 94: 2563–2568.
Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV et al. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 1999; 401: 133–141.
Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS et al. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol 1999; 1: 119–124.
Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron 1999; 24: 143–154.
So CW, So CK, Cheung N, Chew SL, Sham MH, Chan LC . The interaction between EEN and Abi-1, two MLL fusion partners, and synaptojanin and dynamin: implications for leukaemogenesis. Leukemia 2000; 14: 594–601.
Cheung N, So CW, Yam JW, So CK, Poon RY, Jin DY et al. Subcellular localization of EEN/endophilin A2, a fusion partner gene in leukaemia. Biochem J 2004; 383: 27–35.
Yam JW, Jin DY, So CW, Chan LC . Identification and characterization of EBP, a novel EEN binding protein that inhibits Ras signaling and is recruited into the nucleus by the MLL-EEN fusion protein. Blood 2004; 103: 1445–1453.
Liu H, Chen B, Xiong H, Huang QH, Zhang QH, Wnag ZG et al. Functional contribution of EEN to leukemogenic transformation by MLL-EEN fusion protein. Oncogene 2004; 23: 3385–3394.
Sparks AB, Hoffman NG, McConnell SJ, Fowlkes DM, Kay BK . Cloning of ligand targets: systematic isolation of SH3 domain-containing proteins. Nat Biotechnol 1996; 14: 741–744.
Zhang Y, Buchholz F, Muyrers JP, Stewart AF . A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet 1998; 20: 123–128.
Dobson CL, Warren AJ, Pannell R, Forster A, Rabbitts TH . Tumorigenesis in mice with a fusion of the leukaemia oncogene Mll and the bacterial lacZ gene. EMBO J 2000; 19: 843–851.
Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med 2005; 201: 1603–1614.
Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev 1995; 9: 1753–1765.
Antonchuk J, Sauvageau G, Humphries RK . HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 2002; 109: 39–45.
Polo S, Pece S, Di Fiore PP . Endocytosis and cancer. Curr Opin Cell Biol 2004; 16: 156–161.
Bache KG, Slagsvold T, Stenmark H . Defective downregulation of receptor tyrosine kinases in cancer. EMBO J 2004; 23: 2707–2712.
Acknowledgements
This study is supported by research grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. HKU7269/02M, HKU1/98C and HKU2/02M). We thank Cary So for technical support, Jian Dong Huang for help with construction of targeting vector, Florence Loong for help with histology and Chi Leong So, Keith Leung, Christine Ng and Carly Lam for help with gene targeting in ES cells and generation of mutant mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, C., Sham, M., So, C. et al. The Mll-Een knockin fusion gene enhances proliferation of myeloid progenitors derived from mouse embryonic stem cells and causes myeloid leukaemia in chimeric mice. Leukemia 20, 1829–1839 (2006). https://doi.org/10.1038/sj.leu.2404342
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404342