Abstract
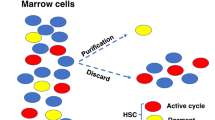
In unperturbed mice, the marrow cell numbers correlate with the stem cell numbers. High levels of long-term marrow engraftment are obtained with infusion of high levels of marrow cells in untreated mice. To address the issue of stem cell competition vs ‘opening space’, knowledge of total murine marrow cellularity and distribution of stem and progenitor cells are necessary. We determined these parameters in different mouse strains. Total cellularity in BALB/c mice was 530±20 million cells; stable from 8 weeks to 1 year of age. C57BL/6J mice had 466±48 million marrow cells. Using these data, theoretical models of infused marrow (40 million cells) replacing or adding to host marrow give chimerism values of 7.5 and 7.0%, respectively; the observed 8-week engraftment of 40 million male BALB/c marrow cells into female hosts (72 mice) gave a value of 6.91±0.4%. This indicates that syngeneic engraftment is determined by stem cell competition. Our studies demonstrate that most marrow cells, progenitors and engraftable stem cells are in the spine. There was increased concentration of progenitors in the spine. Total marrow harvest for stem cell purification and other experimental purposes was both mouse and cost efficient with over a four-fold decrease in animal use and a financial saving.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lambert JF, Colvin GA, Zhong S, Wang H, D'Hondt L, Abedi M et al. H2-mismatched transplantation with repetitive cell infusions and CD40 ligand antibody infusions without myeloablation. Br J Haematol 2002; 119: 155–163.
Bradley MB, Sattler RM, Raftopoulos H, Ward M, Grossman IR, Townes TM et al. Correction of phenotype in a thalassemia mouse model using a nonmyeloablative marrow transplantation regimen. Biol Blood Marrow Transplant 2002; 8: 453–461.
Quesenberry PJ, Stewart MF, Peters S, Nillson S, Ramshaw H, Rao S et al. Engraftment of hematopoietic stem cells in nonmyeloablated and myeloablated hosts. Stem Cells 1997; 15 (Suppl 1): 167–169.
Stewart FM, Crittenden RB, Lowry PA, Pearson-White S, Quesenberry PJ . Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood 1993; 81: 2566–2571.
Quesenberry PJ, Ramshaw H, Crittenden RB, Stewart FM, Rao S, Peters S et al. Engraftment of normal murine marrow into nonmyeloablated host mice. Blood Cells 1994; 20: 348–350.
Rao SS, Peters SO, Crittenden RB, Stewart FM, Ramshaw HS, Quesenberry PJ . Stem cell transplantation in the normal nonmyeloablated host: relationship between cell dose, schedule, and engraftment. Exp Hematol 1997; 25: 114–121.
Ramshaw HS, Crittenden RB, Dooner M, Peters SO, Rao SS, Quesenberry PJ . High levels of engraftment with a single infusion of bone marrow cells into normal unprepared mice. Biol Blood Marrow Transplant 1995; 1: 74–80.
Stewart FM, Zhong S, Lambert JF, Colvin GA, Abedi M, Dooner MS et al. Host marrow stem cell potential and engraftability at varying times after low-dose whole-body irradiation. Blood 2001; 98: 1246–1251.
Sykes M, Spitzer TR . Non-myeloblative induction of mixed hematopoietic chimerism: application to transplantation tolerance and hematologic malignancies in experimental and clinical studies. Cancer Treat Res 2002; 110: 79–99.
van den Bos C, Kieboom D, Wagemaker G . Correction of murine beta-thalassemia by partial bone marrow chimerism: selective advantage of normal erythropoiesis. Bone Marrow Transplant 1993; 12: 9–13.
Mardiney III M, Malech HL . Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood 1996; 87: 4049–4056.
Stewart FM, Zhong S, Wuu J, Hsieh C, Nilsson SK, Quesenberry PJ . Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood 1998; 91: 3681–3687.
Micklem HS, Clarke CM, Evans EP, Ford CE . Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation 1968; 6: 299–302.
Takada A, Takada Y, Ambrus JL . Proliferation of donor spleen and bone-marrow cells in the spleens and bone marrows of unirradiated and irradiated adult mice. Proc Soc Exp Biol Med 1971; 136: 222–226.
Takada Y, Takada A . Proliferation of donor hematopoietic cells in irradiated and unirradiated host mice. Transplantation 1971; 12: 334–338.
Brecher G, Ansell JD, Micklem HS, Tjio JH, Cronkite EP . Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc Natl Acad Sci USA 1982; 79: 5085–5087.
Saxe DF, Boggs SS, Boggs DR . Transplantation of chromosomally marked syngeneic marrow cells into mice not subjected to hematopoietic stem cell depletion. Exp Hematol 1984; 12: 277–283.
Weiss L . The hematopoietic microenvironment of the bone marrow: an ultrastructural study of the stroma in rats. Anat Rec 1976; 186: 161–184.
Kriegler AB, Verschoor SM, Bernardo D, Bertoncello I . The relationship between high proliferative potential colony-forming cells in mouse bone marrow. Exp Hematol 1994; 22: 432–440.
McNiece IK, Bertoncello I, Kriegler AB, Quesenberry PJ . Colony-forming cells with high proliferative potential (HPP-CFC). Int J Cell Cloning 1990; 8: 146–160.
Riedy MC, Muirhead KA, Jensen CP, Stewart CC . Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell population. Cytometry 1991; 12: 133–139.
Wilcoxon F . Individual comparisons by ranking methods. Biometrics 1945; 1: 80–83.
Armitage PE, Berry G . Statistical Methods in Medical Research, 3rd edn. Oxford Blackwell Science Ltd, 1994.
Cuzick J . A Wilcoxon-type test for trend. Stat Med 1985; 4: 87–90.
Boggs DR . The total marrow mass of the mouse: a simplified method of measurement. Am J Heme 1984; 16: 277–286.
Vácha J, Hola J, Dungel J, Znojil V . The distribution of erythropoiesis over the various anatomical regions of the erythropoietic system in some inbred strains of mice. Exp Heme 1982; 10: 768–773.
Ramshaw HS, Crittenden RB, Dooner M, Peters SO, Rao SS, Quesenberry PJ . High levels of engraftment with a single infusion of bone marrow cells into normal unprepared mice. Biol Blood Marrow Transplant 1995; 1: 74–80.
Blomberg M, Rao S, Reilly J, Tiarks C, Peters S, Kittler E et al. Repetitive bone marrow transplantation in nonmyeloablated recipients. Exp Hematol 1998; 26: 320–324.
Cronkite EP, Bullis JE, Brecher G . Marrow transfusions increase pluripotential stem cells in normal hosts. Exp Hematol 1985; 13: 802–805.
van Os R, Sheridan TM, Robinson S, Drukteinis D, Ferrara JL, Mauch PM . Immunogenicity of Ly5 (CD45)-antigens hampers long-term engraftment following minimal conditioning in a murine bone marrow transplantation model. Stem Cells 2001; 19: 80–87.
Boggs DR, Patrene KD . Marrow mass and distribution in murine skeletons cleaned by beetles as compared to cut up carcasses and a further simplification of the latter technique. Am J Hematol 1986; 21: 49–55.
Acknowledgements
This work was supported by National Institutes of Health sponsored grants: P01 HL56920-03, P01 DK50222-03, R01 DK27424-18, R01 DK60084, R01 DK60090-01A1, and K08 DK64980-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colvin, G., Lambert, JF., Abedi, M. et al. Murine marrow cellularity and the concept of stem cell competition: geographic and quantitative determinants in stem cell biology. Leukemia 18, 575–583 (2004). https://doi.org/10.1038/sj.leu.2403268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403268
Keywords
This article is cited by
-
Ablative radiotherapy improves survival but does not cure autochthonous mouse models of prostate and colorectal cancer
Communications Medicine (2023)
-
Humanized mice for investigating sustained Plasmodium vivax blood-stage infections and transmission
Nature Communications (2022)
-
Brain motor and fear circuits regulate leukocytes during acute stress
Nature (2022)
-
Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection
Nature Immunology (2020)
-
A revisionist history of adult marrow stem cell biology or ‘they forgot about the discard’
Leukemia (2017)