Abstract
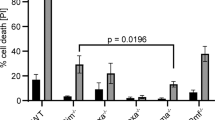
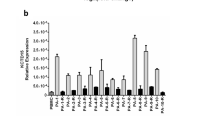
Bmf is a BH3-only Bcl-2 family member that is normally sequestered to myosin V motors by binding to the dynein light chain 2 (DLC2). Certain damage signals release Bmf, which then binds prosurvival Bcl-2 proteins and triggers apoptosis. Here, two novel isoforms of human Bmf, Bmf-II and Bmf-III, were identified and cloned from cDNA derived from B-chronic lymphocytic leukemia (B-CLL) cells. Bmf-II and Bmf-III were characterized as two splice variants, lacking the BH3 domain but retaining the DLC2 binding domain. Bmf (here called Bmf-I) expression in HeLa cells induced apoptosis and reduced colony formation in contrast to Bmf-II and Bmf-III, which had no effect on apoptosis and instead increased colony formation. While bmf-I mRNA was expressed in many cell types, expression was higher in B lymphoid cells and bmf-II and bmf-III were mainly detected in B-CLL and normal B cells. bmf-I mRNA was upregulated in normal and leukemic B cells, while bmf-III mRNA was downregulated only in B-CLL cells by serum deprivation. We show that Bmf is regulated by transcriptional activation and alternative splicing and conclude that the relative levels of Bmf isoforms may have a role in regulating growth and survival in B cells and leukemic B-CLL cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cory S, Adams JM . The BCL-2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2: 647–656.
Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M . Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 1997; 275: 983–986.
O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S . Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J 1998; 17: 384–395.
Bouillet P, Strasser A . BH3-only proteins – evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci 2002; 115: 1567–1574.
Huang DCS, Strasser A . BH3-only proteins – essential initiators of apoptotic cell death. Cell 2000; 103: 839–842.
Puthalakath H, Strasser A . Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 2002; 9: 505–512.
Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE . Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001; 293: 1829–1832.
Puthalakath H, Huang DCS, O'Reilly LA, King SM, Strasser A . The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 1999; 3: 287–296.
Mount SM . A catalogue of splice junction sequences. Nucleic Acids Res 1982; 10: 459–472.
Collins RJ, Verschuer LA, Harmon BV, Prentice RL, Pope JH, Kerr JF . Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol 1989; 71: 343–350.
Aguilar-Santelises M, Rottenberg ME, Lewin N, Mellstedt H, Jondal M . Bcl-2, Bax and p53 expression in B-CLL in relation to in vitro survival and clinical progression. Int J Cancer 1996; 69: 114–119.
Boillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F . Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738.
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H . BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 2002; 415: 922–926.
Dijkers PF, Medema RH, Lammers J-WJ, Koenderman L, Coffer PJ . Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol 2000; 10: 1201–1204.
Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A . Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol Cell Biol 2001; 21: 854–864.
Lei K, Davis RJ . JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax dependent apoptosis. Proc Natl Acad Sci 2003; 100: 2432–2437.
Villunger A, Scott C, Bouillet P, Strasser A . Essential role for the BH3-only protein Bim, but redundant roles for Bax, Bcl-2 and Bcl-w in the control of granulocyte survival. Blood 2003; 101: 2393–2400.
Caligaris-Cappio F, Cignetti A, Granziero L, Ghia P . Chronic lymphocytic leukemia: a model for investigating potential new targets for the therapy of indolent lymphomas. Best Pract Res Clin Haematol 2002; 15: 563–575.
Binet JL, Plunkett W, Robertson B, Merle-Beral H, Mentz F, Hoffbrand AV, Panayiotidis P . What does apoptosis mean in CLL? Leukemia Lymphoma 1996; 22 (Suppl 2): 47–52.
Consoli U, El-Tounsi I, Sandoval A, Snell V, Kleine HD, Brown W . Differential induction of apoptosis by fludarabine monophosphate in leukemic B and normal T cells in chronic lymphocytic leukemia. Blood 1998; 91: 1742–1748.
Ricciardi MR, Petrucci MT, Gregorj C, Ariola C, Lemoli RM, Fogli M . Reduced susceptibility to apoptosis correlates with kinetic quiescence in disease progression of chronic lymphocytic leukaemia. Br J Haematol 2001; 113: 391–399.
Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM . Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell 2001; 1: 645–653.
Pepper C, Hoy T, Bentley P . Elevated Bcl-2/Bax are a consistent feature of apoptosis resistance in B-cell chronic lymphocytic leukemia and are correlated with in vivo chemoresistance. Leukemia Lymphoma 1998; 28: 355–361.
Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S . Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood 1998; 91: 3379–3389.
Acknowledgements
This work was supported by funds from the Swedish Cancer Society, The Swedish Medical Association and the Karolinska Institutet, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morales, A., Olsson, A., Celsing, F. et al. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia 18, 41–47 (2004). https://doi.org/10.1038/sj.leu.2403183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403183
Keywords
This article is cited by
-
Dynein light chain binding determines complex formation and posttranslational stability of the Bcl-2 family members Bmf and Bim
Cell Death & Differentiation (2020)
-
Heterogeneous Nuclear Ribonucleoprotein F Mediates Insulin Inhibition of Bcl2-Modifying Factor Expression and Tubulopathy in Diabetic Kidney
Scientific Reports (2019)
-
How cell death shapes cancer
Cell Death & Disease (2015)
-
Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death
Cell Death & Disease (2013)
-
BH3-only protein Bmf mediates apoptosis upon inhibition of CAP-dependent protein synthesis
Cell Death & Differentiation (2010)