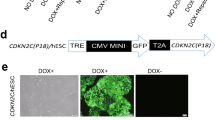
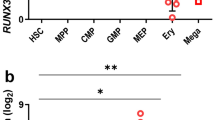
Abstract
The Wilms tumor gene (WT1) encodes a zinc-finger containing transcription factor present in primitive hematopoietic progenitor cells. WT1 is also highly expressed in most cases of acute myeloid leukemia. Moreover, WT1 can interfere with induced differentiation of leukemic cell lines. These data suggest a function of WT1 in the maintenance of a primitive phenotype and a role in leukemogenesis by interfering with differentiation, prompting us to investigate its function in human hematopoietic progenitor cells. By retroviral transfer, human CD34+ cord blood progenitor cells were transduced with a vector encoding either of two splicing variants of WT1, with or without the KTS insert in the zinc-finger domain, linked to expression of green fluorescent protein (GFP) via an internal ribosomal entry site. When compared to cells transduced with vector containing GFP only, WT1 expressing cells showed strongly reduced colony formation in methylcellulose and inhibited proliferation in suspension culture, with no apparent reduction in viability. Cell cycle phase distribution was not affected by WT1 expression. No signs of impaired differentiation, as judged by the surface markers CD11b, CD14 and glycophorin were detected. In contrast to the results with human CD34+ progenitor cells, the proliferation of murine bone marrow cells was not significantly affected by WT1, consistent with previous data. We conclude that forced expression of WT1 in highly enriched human hematopoietic progenitor cells leads to strong anti-proliferative effects but is compatible with induced maturation of these cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH . Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus Cell 1990 60: 509–520
Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA . Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping Nature 1990 343: 774–778
Haber DA, Buckler AJ, Glaser T, Call KM, Pelletier J, Sohn RL, Douglass EC, Housman DE . An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor Cell 1990 61: 1257–1269
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R . WT-1 is required for early kidney development Cell 1993 74: 679–691
Herzer U, Crocoll A, Barton D, Howells N, Englert C . The Wilms tumor suppressor gene wt1 is required for development of the spleen Curr Biol 1999 9: 837–840
Rauscher FJd . The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor FASEB J 1993 7: 896–903
Reddy JC, Licht JD . The WT1 Wilms’ tumor suppressor gene: how much do we really know? Biochim Biophys Acta 1996 1287: 1–28
Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re GG, Garvin AJ, Rosner MR, Haber DA . WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis EMBO J 1995 14: 4662–4675
Cook DM, Hinkes MT, Bernfield M, Rauscher FJ 3rd . Transcriptional activation of the syndecan-1 promoter by the Wilms’ tumor protein WT1 Oncogene 1996 13: 1789–1799
Lee SB, Huang K, Palmer R, Truong VB, Herzlinger D, Kolquist KA, Wong J, Paulding C, Yoon SK, Gerald W, Oliner JD, Haber DA . The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin Cell 1999 98: 663–673
Hosono S, Gross I, English MA, Hajra KM, Fearon ER, Licht JD . E-cadherin is a WT1 target gene J Biol Chem 2000 275: 10943–10953
Haber DA, Sohn RL, Buckler AJ, Pelletier J, Call KM, Housman DE . Alternative splicing and genomic structure of the Wilms tumor gene WT1 Proc Natl Acad Sci USA 1991 88: 9618–9622
Brieger J, Weidmann E, Fenchel K, Mitrou PS, Hoelzer D, Bergmann L . The expression of the Wilms’ tumor gene in acute myelocytic leukemias as a possible marker for leukemic blast cells Leukemia 1994 8: 2138–2143
Fraizer GC, Patmasiriwat P, Zhang X, Saunders GF . Expression of the tumor suppressor gene WT1 in both human and mouse bone marrow (letter) Blood 1995 86: 4704–4706
Maurer U, Brieger J, Weidmann E, Mitrou PS, Hoelzer D, Bergmann L . The Wilms’ tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro Exp Hematol 1997 25: 945–950
Miwa H, Beran M, Saunders GF . Expression of the Wilms’ tumor gene (WT1) in human leukemias Leukemia 1992 6: 405–409
Inoue K, Sugiyama H, Ogawa H, Nakagawa M, Yamagami T, Miwa H, Kita K, Hiraoka A, Masaoka T, Nasu K et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia Blood 1994 84: 3071–3079
Inoue K, Tamaki H, Ogawa H, Oka Y, Soma T, Tatekawa T, Oji Y, Tsuboi A, Kim EH, Kawakami M, Akiyama T, Kishimoto T, Sugiyama H . Wilms’ tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells Blood 1998 91: 2969–2976
Maurer U, Weidmann E, Karakas T, Hoelzer D, Bergmann L . Wilms tumor gene (wt1) mRNA is equally expressed in blast cells from acute myeloid leukemia and normal CD34+ progenitors (letter) Blood 1997 90: 4230–4232
Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y, Miyake S, Tamaki H, Oji Y, Yamagami T, Tatekawa T, Soma T, Kishimoto T, Sugiyama H . Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia Blood 1997 89: 1405–1412
Inoue K, Ogawa H, Yamagami T, Soma T, Tani Y, Tatekawa T, Oji Y, Tamaki H, Kyo T, Dohy H, Hiraoka A, Masaoka T, Kishimoto T, Sugiyama H . Long-term follow-up of minimal residual disease in leukemia patients by monitoring WT1 (Wilms tumor gene) expression levels Blood 1996 88: 2267–2278
Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, Hoelzer D . High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome Blood 1997 90: 1217–1225
Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, Aozasa K, Kishimoto T, Sugiyama H . Cancer immunotherapy targeting Wilms’ tumor gene WT1 product J Immunol 2000 164: 1873–1880
Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, Stauss HJ . Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1 Blood 2000 95: 2198–2203
Gaiger A, Reese V, Disis ML, Cheever MA . Immunity to WT1 in the animal model and in patients with acute myeloid leukemia Blood 2000 96: 1480–1489
King Underwood L, Renshaw J, Pritchard-Jones K . Mutations in the Wilms’ tumor gene WT1 in leukemias Blood 1996 87: 2171–2199
King-Underwood L, Pritchard-Jones K . Wilms’ tumor (WT1) gene mutations occur mainly in acute myeloid leukemia and may confer drug resistance Blood 1998 91: 2961–2968
Sekiya M, Adachi M, Hinoda Y, Imai K, Yachi A . Downregulation of Wilms’ tumor gene (wt1) during myelomonocytic differentiation in HL60 cells Blood 1994 83: 1876–1882
Phelan SA, Lindberg C, Call KM . Wilms’ tumor gene, WT1, mRNA is down-regulated during induction of erythroid and megakaryocytic differentiation of K562 cells Cell Growth Differ 1994 5: 677–686
Svedberg H, Chylicki K, Baldetorp B, Rauscher F Jr, Gullberg U . Constitutive expression of the Wilms’ tumor gene (WT1) in the leukemic cell line U937 blocks parts of the differentiation program Oncogene 1998 16: 925–932
Deuel TF, Guan LS, Wang ZY . Wilms’ tumor gene product WT1 arrests macrophage differentiation of HL-60 cells through its zinc-finger domain Biochem Biophys Res Commun 1999 254: 192–196
Smith SI, Weil D, Johnson GR, Boyd AW, Li CL . Expression of the Wilms’ tumor suppressor gene, WT1, is upregulated by leukemia inhibitory factor and induces monocytic differentiation in M1 leukemic cells Blood 1998 91: 764–773
Smith SI, Down M, Boyd AW, Li CL . Expression of the Wilms’ tumor suppressor gene, WT1, reduces the tumorigenicity of the leukemic cell line M1 in C.B-17 scid/scid mice Cancer Res 2000 60: 808–814
Tsuboi A, Oka Y, Ogawa H, Elisseeva OA, Tamaki H, Oji Y, Kim EH, Soma T, Tatekawa T, Kawakami M, Kishimoto T, Sugiyama H . Constitutive expression of the Wilms’ tumor gene WT1 inhibits the differentiation of myeloid progenitor cells but promotes their proliferation in response to granulocyte-colony stimulating factor (G-CSF) Leuk Res 1999 23: 499–505
Hawley RG . High-titer retroviral vectors for efficient transduction of functional genes into murine hematopoietic stem cells Ann NY Acad Sci 1994 716: 327–330
Ghattas IR, Sanes JR, Majors JE . The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos Mol Cell Biol 1991 11: 5848–5859
Ory DS, Neugeboren BA, Mulligan RC . A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes Proc Natl Acad Sci USA 1996 93: 11400–11406
Luens KM, Travis MA, Chen BP, Hill BL, Scollay R, Murray LJ . Thrombopoietin, kit ligand, and flk2/flt3 ligand together induce increased numbers of primitive hematopoietic progenitors from human CD34+Thy-1+Lin− cells with preserved ability to engraft SCID-hu bone Blood 1998 91: 1206–1215
Ramsfjell V, Bryder D, Bjorgvinsdottir H, Kornfalt S, Nilsson L, Borge OJ, Jacobsen SE . Distinct requirements for optimal growth and in vitro expansion of human CD34(+)CD38(−) bone marrow long-term culture-initiating cells (LTC-IC), extended LTC-IC, and murine in vivo long-term reconstituting stem cells Blood 1999 94: 4093–4102
Chylicki K, Ehinger M, Svedberg H, Bergh G, Olsson I, Gullberg U . p53-mediated differentiation of the erythroleukemia cell line K562 Cell Growth Differ 2000 11: 315–324
Ellisen LW, Carlesso N, Cheng T, Scadden DT, Haber DA . The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells EMBO J 2001 20: 1897–1909
Englert C, Maheswaran S, Garvin AJ, Kreidberg J, Haber DA . Induction of p21 by the Wilms’ tumor suppressor gene WT1 Cancer Res 1997 57: 1429–1434
Murata Y, Kudoh T, Sugiyama H, Toyoshima K, Akiyama T . The Wilms tumor suppressor gene WT1 induces G1 arrest and apoptosis in myeloblastic leukemia M1 cells FEBS Lett 1997 409: 41–55
English MA, Licht JD . Tumor-associated WT1 missense mutants indicate that transcriptional activation by WT1 is critical for growth control J Biol Chem 1999 274: 13258–13263
Svedberg H, Chylicki K, Gullberg U . Downregulation of Wilms’ tumor gene (WT1) is not a prerequisite for erythroid or megakaryocytic differentiation of the leukemic cell line K562 Exp Hematol 1999 27: 1057–1062
Buckler AJ, Pelletier J, Haber DA, Glaser T, Housman DE . Isolation, characterization, and expression of the murine Wilms’ tumor gene (WT1) during kidney development Mol Cell Biol 1991 11: 1707–1712
Werner H, Shen Orr Z, Rauscher FJr, Morris JF, Roberts CT Jr, LeRoith D . Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression Mol Cell Biol 1995 15: 3516–3522
Wagner KD, Wagner N, Sukhatme VP, Scholz H . Activation of vitamin D receptor by the Wilms’ tumor geneproduct mediates apoptosis of renal cells J Am Soc Nephrol 2001 12: 1188–1196
Borner C . Diminished cell proliferation associated with the death-protective activity of Bcl-2 J Biol Chem 1996 271: 12695–12698
Vairo G, Innes KM, Adams JM . Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival Oncogene 1996 13: 1511–1519
Tabor CW, Tabor H . Polyamines Annu Rev Biochem 1984 53: 749–790
Li RS, Law GL, Seifert RA, Romaniuk PJ, Morris DR . Ornithine decarboxylase is a transcriptional target of tumor suppressor WT1 Exp Cell Res 1999 247: 257–269
Acknowledgements
The authors wish to thank the staff at the Department of Obstetrics and Gynecology, Malmö University Hospital for invaluable help with the collection of cord blood samples. We thank Tor Olofsson for important and professional advice and the staff at ICH for help with the the flow cytometry analyses, Sverker Segrén for performing the flow cytometry cell sorting, and Thomas Relander for help with generation of retroviral vectors. The scientific advice and critical review of the manuscript by Inge Olsson is acknowledged. This work was supported by grants from the Swedish Cancer Society, the Swedish Medical Research Council (project No. 11546), the Swedish Childhood Cancer Foundation, the Bergquist Foundation, the Berta Kamprad Foundation, the Crafoord Foundation, the Georg Danielsson Foundation, the Gunnar Nilsson Cancer Foundation, the John Persson Foundation, the Thelma Zoega Foundation, the Greta and Johan Kock Foundation, the John and Augusta Persson Foundation, the Tobias Foundation, the Österlund Foundation, and from Funds of Lund University Hospital.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Svedberg, H., Richter, J. & Gullberg, U. Forced expression of the Wilms tumor 1 (WT1) gene inhibits proliferation of human hematopoietic CD34+ progenitor cells. Leukemia 15, 1914–1922 (2001). https://doi.org/10.1038/sj.leu.2402303
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402303
Keywords
This article is cited by
-
Wilms' tumours: about tumour suppressor genes, an oncogene and a chameleon gene
Nature Reviews Cancer (2011)
-
The Wilms’ tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis
Leukemia (2007)
-
A tumor suppressor and oncogene: the WT1 story
Leukemia (2007)
-
RNA-based gene transfer for adult stem cells and T cells
Leukemia (2004)
-
Transcriptional activation of c-myc proto-oncogene by WT1 protein
Oncogene (2004)