Abstract
Activation of the platelet-derived growth factor (PDGF)/PDGF beta receptor (PDGFβR) axis has a critical role in liver fibrosis. However, the mechanisms that regulate the PDGF signaling are yet to be elucidated. The present study demonstrates that paired related homeobox protein 1 (Prrx1) is involved in PDGF-dependent hepatic stellate cell (HSCs) migration via modulation of the expression of metalloproteinases MMP2 and MMP9. PDGF elevated the level of Prrx1 through the activation of ERK/Sp1 and PI3K/Akt/Ets1 pathways. In vivo, an adenoviral-mediated Prrx1 shRNA administration attenuated liver fibrosis in thioacetamide-induced fibrotic models. These studies reveal a role of Prrx1 as a modulator of PDGF-dependent signaling in HSCs, and inhibiting its expression may offer a therapeutic approach for hepatic fibrosis.
Similar content being viewed by others
Main
Progressive liver fibrosis is a common consequence of chronic hepatic injury and is characterized by excessive accumulation of extracellular matrix (ECM).1 The activation of hepatic stellate cells (HSCs) is the key event during hepatic fibrogenesis. As a response to continued liver damage, HSCs transform from a quiescent state into fibrogenic, proliferative, contractile, and α-smooth muscle actin (α-SMA)-positive myofibroblast-like phenotype. This transformation is also accompanied by an abundant production of extracellular matrix (ECM) and secretion of high levels of cytokines, such as transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF).2, 3
PDGF is a potent mitogen and chemotactic cytokine that is frequently elevated in a fibrotic liver.4, 5, 6 Previous studies have shown that the overexpression of PDGFs could drive the proliferation, motility, and chemotaxis of HSCs,5, 6 whereas the loss of PDGFR or PDGFβ leads to embryonic lethality in mice.7, 8 In addition, the specific depletion of PDGF-βR in HSCs reduces the liver injury and fibrosis in mice.9
The pair-related homeobox transcription factor, Prrx1, has two alternative splicing isoforms, Prrx1a and Prrx1b. These isoforms are primarily different at the C-terminal end and interact with other factors to enhance the specific DNA binding activity in a similar manner.10, 11 Under physiological conditions, Prrx1 has crucial roles in embryonic development. Prrx1−/− mice suffer perinatal death along with craniofacial defects and limb shortening.12, 13, 14 Prrx1 is also involved in the self-renewal in neural stem cells and pancreatic regeneration,15, 16 and its expression promotes tenascin C-dependent fibroblast migration.17 Recent evidence show that Prrx1 can induce the epithelial-mesenchymal transition (EMT) in pancreatic and breast cancer cells. Furthermore, the knockdown of Prrx1 manifests stem cell-like features in cancer cells.16, 18 Prrx1 is also implicated in the regulation of mesenchymal cell fate. Previous studies demonstrated that Prrx1 was highly upregulated in the fibrotic liver and was responsible for the synthesis of procollagen and transforming growth factor beta 3 (TGFB3) in the activated HSCs.19, 20
In this study, we demonstrated for the first time that Prrx1 acts as a critical downstream molecule in PDGF signaling pathway and has a major role in the recruitment of PDGF-dependent HSCs. These discoveries provide new insights into the mechanisms of PDGF-regulated processes; also it might serve as a novel target for the therapeutic intervention in fibrotic liver.
Materials and methods
Reagents
Human recombinant PDGF-BB was purchased from Peprotech (Rocky Hill, CT, USA). Compounds such as U0126 and LY294002 were obtained from Sigma (St Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Gibco (Carlsbad, CA, USA) and fibronectin from Corning (New York, NY, USA).
Isolation and Culture of HSCs
Primary HSCs were isolated from male Sprague Dawley rats (SD; 200–250 g) by the pronase–collagenase perfusion in situ before density gradient centrifugation on an OptiPrep gradient, as described previously.21 The primary cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% FBS. The passaged cells were grown in DMEM with 10% of FBS; the rat primary HSCs (R-HSC) were used for up to four passages. The human hepatic stellate cell line, LX-2, obtained from the Institute of Liver Diseases, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, (Wuhan, China) was cultured in DMEM with 10% FBS.
siRNA, Plasmids, Virus, and Cell Transfection
Small interfering RNA (siRNA) duplexes targeting Prrx1, Sp1, and Ets1, as well as scrambled siRNA (NC; Supplementary Table 1), were synthesized by RiboBio (Guangzhou, China). Human Prrx1a (CMV-MCS-3FLAG-SV40-Neomycin) and Prrx1b plasmids, the control vector, an adenoviral vector containing shRNA targeting mouse Prrx1 (AdshPrrx1, targeted sites: 5′-CCAACAGCATTGCCAACCT-3′), and the control adenovirus (AdshNC) were purchased from Genechem (Shanghai, China). The HSCs were transfected with indicated siRNA or plasmids using Lipofectamine 2000 reagent (Carlsbad, CA, USA) according to the standard protocols, and the knockdown efficiency was assessed by real-time PCR and western blot. All the assays were performed at 72–96 h after transfection.
Western Blot and Co-Immunoprecipitations
Following standard electrophoresis, the membranes were probed overnight at 4 °C with primary antibodies listed in Supplementary Table 2. Subsequently, the membranes were incubated with anti-mouse or -rabbit IgG (1:3000; Abbkine, CA, USA), and the signals were detected using an ECL assay kit (Amersham, Buckinghamshire, UK).
For co-immunoprecipitations, the cells were lysed with 1% NP40 NET buffer (Promoter Company, China), the supernatants were incubated overnight at 4 °C with protein G-Sepharose beads (Sigma) conjugated with rabbit anti-Flagor anti-IgG antibody, and then the samples were prepared for western blot assay. For western blot, the membrane was incubated overnight at 4 °C with primary antibodies Sp1, Ets1, Flag, and Prrx1, followed by anti-mouse or -rabbit IgG (1:3000; Sigma, CA, USA), and the signals were detected with an ECL assay kit (Amersham).
Patients Liver Samples
Informed consent was obtained from the tissue donors before collecting a total of 20 fibrotic liver samples from surgical resections in patients with liver hemangioma at the Tongji Hospital in 2015. The procedure of collection of the human samples was approved by the Ethics Committee of the Tongji Hospital, Huazhong University of Science and Technology, and the study was conducted according to the Declaration of Helsinki principles. The liver tissues were fixed in 4% buffered paraformaldehyde and embedded in paraffin for immunostaining.
Animal Fibrosis Models and Adenoviral Delivery In Vivo
CCl4-induced-fibrosis models: male C57 mice, 7–10-week-old, were intraperitoneally (0.5 μl/g of body weight, two times/week) injected with 25% CCl4 (carbon tetrachloride) to induce chronic liver injury, whereas the control mice were administered only olive oil. The injections were continued for 0, 4, and 8 weeks, respectively (n=5) to establish the model for fibrosis with different degrees of severity.
TAA-induced-fibrosis models: male Sprague Dawley (SD) rats, weighing about 300 g, were purchased from the Department of Experimental Animals of Tongji Medical College. The animals (n=8) were treated with thioacetamide (TAA solubilized in saline, 200 mg/kg body weight; Ding Guo, China) by the intraperitoneal injection two times/week for 4, 8, or 12 weeks, respectively, to induce chronic liver injury; the rats were killed 2 days after the final dose. The control group received an equivalent amount of saline alone (n=8).
Adenoviral delivery in vivo: hepatic fibrosis was induced in rats by intraperitoneal injection of TAA (200 mg/kg body weight) two times/week for 6 weeks. Subsequently, each animal received 5 × 109 PFU of Prrx1 shRNA (AdshPrrx1) or control adenovirus vector (AdshNC) via the tail vein, one time/week and was repetitively administered TAA for 2 weeks.
The liver tissues and serum were either snap-frozen at −80 °C or fixed in 4% buffered paraformaldehyde for immunostaining. All animal studies were performed in accordance with the national and international guidelines. The protocol was approved by the Ethics Committee of Animal Experiments of Tongji Medical College and monitored by the Department of Experimental Animals of the Tongji Medical College.
Migration Assay
HSCs transfected with Prrx1 siRNA in the presence or absence of PDGF-BB (20 ng/ml) for 24 h were resuspended in low-serum medium and seeded in the upper chamber (20 000 cells/well) of the polycarbonate membrane transwell inserts (8 μm pore size; Corning, China). The bottom chambers of the system were filled with serum-free medium containing PDGF (10 ng/ml). After incubation for 48 h, the polycarbonate filter was removed and the cells that had migrated to the lower chamber were stained with crystal violet and counted using a microscope; four fields/well were recorded, and the mean number of cells/field was calculated.
In Vitro Wound-Repair Assay
The LX-2 cells were seeded in six-well plates overnight to allow 50% confluency. Subsequently, the cells were transfected with Prrx1 siRNA and control siRNA using Lipofectamine 2000 according to the manufacturer’s instructions. After 24 h, the growth medium was replaced with serum-free medium supplemented with human recombinant PDGF-BB (20 ng/ml). For wound assays, the cell monolayer was scratched using a sterile 200 μl tip after another 24 h. The cell growth in the denuded area was assessed after 24 h, and six images from each sample were captured (× 10 magnification). Cell migration was quantified as the percentage of the wound-healed area.
Adhesion Experiments
The 96-well plates were coated with fibronectin (10 μg/ml) at 37 °C overnight. The plates were washed with PBS, blocked with 5% BSA for 1 h at room temperature, and washed again with PBS. NC- or Prrx1 siRNA-transfected LX-2 cells or primary rat HSCs (4000 cells/well) were incubated in serum-free medium for 2 h at 37 °C. The non-adherent cells were removed, and the others were fixed with 4% paraformaldehyde for 15 min, stained with DAPI, and images captured by a fluorescent microscope for analysis. In addition, we also assessed the adherent cells by incubating them with CCK-8 reagent (Promoter Biotechnology, Wuhan, China) for 3 h, following which, the OD was measured on a multimode reader.
Statistical Analysis
All the experiments were performed in triplicate unless otherwise specified. The data were presented as mean±s.d. Statistical analyses were performed by Student’s t-test using Prism 5.0 (GraphPad Software, La Jolla, CA, USA). A value of P<0.05 was considered statistically significant.
Results
Prrx1 Expression in HSC Increases During Liver Injury and Correlates with the Level of Fibrosis
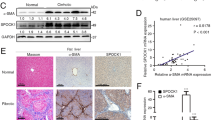
To confirm the role of Prrx1 in liver fibrosis, we assessed the expression of Prrx1 in two animal models of induced fibrosis: the rats treated with TAA and the mice were treated with CCl4. The mRNA and protein levels of Prrx1 were upregulated in TAA-induced fibrotic livers as compared with the normal livers (Figure 1a–c). A similar pattern was also observed in chronic CCl4-induced liver fibrosis in mouse liver where the Prrx1 expression appeared higher than in the uninjured mouse liver (Figure 1d–f).
Increased expression of Prrx1 in fibrotic models. (a) The expression of α-SMA was assessed by immunohistochemistry. HE and Masson’s trichrome staining were used to examine the pathological alterations and collagen deposition in TAA-treated rats. (b and c) The mRNA and protein levels of Prrx1 and α-SMA in the fibrotic livers from TAA-treated rats were detected by real-time PCR (n=7) and western blot (n=2) in each group, respectively. (d) HE and Masson’s trichrome staining were used to examine the pathological alterations and collagen deposition in CCl4-treated mice. (e and f) The mRNA and protein levels of Prrx1 and α-SMA in the fibrotic livers from CCl4-treated rats were detected by real-time PCR (n=5) and western blot (n=2) in each group, respectively. GAPDH was used as a loading control. *P<0.05, **P<0.01 vs normal control group by Student’s t-test.
To further investigate the localization of Prrx1 in injured livers, we performed immunofluorescence double staining. The results revealed that activated HSCs (α-SMA+ cells) strongly expressed Prrx1 in surgically resected fibrotic liver samples and that of TAA-treated rats (Figure 2a and Supplementary Figure 1). In order to confirm the identity of the cells, we isolated quiescent rat HSCs and stimulated them in culture. These activated HSCs exhibited a bright staining of Prrx1 in the nucleus (Figure 2b), suggesting that Prrx1 could have a role in HSC activation.
Prrx1 exerted its profibrotic role in HSCs. (a) Immunofluorescence co-staining of Prrx1 (red) and α-SMA (green) in the liver of human cirrhotic tissue. (b) Immunofluorescence staining of Prrx1 and α-SMA in freshly isolated (1 d) and activated (14 d) primary rat HSCs. Rat HSCs were in the quiescent state and had spontaneous blue-green fluorescence on the first day after isolation.
PDGF-BB Induces Prrx1 Expression in HSCs
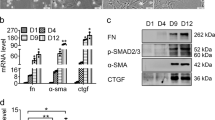
PDGF-BB is primarily a mitogen.2 The increased expression of PDGFβ receptor (PDGF-βR) is a hallmark of HSC activation.22 Thus, we explored whether PDGF-BB could regulate Prrx1 expression in HSCs. A dose-dependent regulation revealed that mRNA and protein levels of Prrx1 increased noticeably after treatment with PDGF-BB at 20 ng/ml for 48 h (Figure 3a and b). Furthermore, PDGF-BB promoted the expression of Prrx1 in a time-dependent manner, and this increase was sustained for 72 h (Figure 3c and d). In addition, the expressions of Sp1 and p-Ets1 increased in response to PDGF-BB treatment and were significantly correlated with the expression of Prrx1 (Figure 3c and d). These data indicated that PDGF-BB directly activates the Prrx1 expression at the transcriptional level.
PDGF-BB-induced Prrx1 expression. (a and c) PDGF-BB induced Prrx1 level in a dose-dependent manner. LX-2 were serum-starved for 24 h and treated with the indicated concentrations of PDGF-BB (0, 5, 10, 15, and 20 ng/ml) with 1% FBS for an additional 48 h. RNA and protein were extracted from the cells. (b and d) PDGF-BB upregulated the Prrx1 expression in a time-dependent manner. LX-2 were serum-starved for 24 h and treated with 15 ng/ml PDGF at the indicated time points (0, 12, 24, 48, and 72 h) before the extraction of mRNA and protein. Prrx1 mRNA level was quantified by real-time PCR; Prrx1, p-Ets1, and Sp1 protein levels were analyzed by western blot analysis. *P<0.05, **P<0.01 vs control group by Student’s t-test.
PDGF-BB Upregulates Prrx1 Expression Through PI3K and ERK Pathways
Next, we sought to investigate the molecular mechanisms underlying the PDGF-BB-induced Prrx1 expression. The canonical downstream effectors for PDGFβR-dependent trophic actions are Akt and ERK.23 The pretreatment with ERK1/2 inhibitor (U0126) or PI3K inhibitor (LY294002) significantly blocked the PDGF-induced upregulation of Prrx1 mRNA and protein level (Figure 4a and b). Notably, the levels of Sp1 and phosphorylated Ets1 were altered, when ERK and Akt pathways were inactivated, respectively (Figure 4b). Hence, we revealed that PDGF-BB induces Prrx1 expression in HSCs via PI3K/Akt and ERK-dependent mechanisms.
PDGF-BB promoted Prrx1 expression by activating ERK/Sp1 and Akt/p-Ets1 in HSCs. (a and b) LX-2 cells were treated with ERK inhibitor (U0126, 25 μM) and PI3K inhibitor (LY294002, 25 μM) for 1 h before PDGF-BB stimulation (15 ng/ml) for an additional 48 h. ERK and Akt signals, p-Ets1, Sp1, and Prrx1 levels were examined by western blot. Prrx1 mRNA level was detected by real-time PCR. *P<0.05 vs PDGF-BB. (c and e) LX-2 cells were transfected with Sp1 siRNA or control siRNA in the presence or absence of PDGF-BB (15 ng/ml) for 48 h. Sp1, Prrx1, MMP2, and cyclinD1 levels were detected by real-time PCR and western blot. (d and f) LX-2 cells were transfected with Ets1 siRNA or control siRNA in the presence or absence of PDGF-BB (15 ng/ml) for 48 h. p-Ets1, Prrx1, MMP2, and cyclinD1 levels were detected by real-time PCR and Western blot. *P<0.05, **P<0.01. (g) LX-2 cells were transfected with Ets1 siRNA, Sp1 siRNA, Ets1 siRNA+Sp1 siRNA, or control siRNA in the presence of PDGF-BB (15 ng/ml) for 48 h. Prrx1 mRNA level was detected by real-time PCR. *P<0.05, **P<0.01. (h and i) LX-2 cells were transfected with Sp1 siRNA (h) or Ets1 siRNA (i). After 24 h, the cells were transfected with Prrx1a, Prrx1b, and control vector plasmids for an additional 48 h, respectively. Western blot analysis was used to examine the expression of Ets1, Sp1, Prrx1, MMP2, and cyclinD1.
Transcription Factor Sp1 and Ets1 have Crucial Roles in Prrx1 Expression Mediated by PDGF-BB
A previous study has demonstrated that PDGF-BB could promote Sp1 expression, nuclear localization, and phosphorylation to increase its transcriptional activity.24 Reportedly, Ets1, as the downstream effector of PI3K signaling pathway, is upregulated by PDGF-BB.25 Our results confirmed that PDGF-BB upregulated the expressions of Sp1 and p-Ets1 similar to that of the Prrx1 level. Therefore, we examined the effect of silencing Sp1 and Ets1 on PDGF-BB-induced Prrx1 expression. The expressions of Sp1 and Ets1 were silenced in LX-2 cells, respectively, before treating with vehicle or PDGF-BB. The results showed that the knockdown of Sp1 or Ets1 dramatically ablated the PDGF-BB-induced Prrx1 mRNA and protein expression. Also, MMP2 and cyclinD1 were downregulated (Figure 4c–f). Compared with targeting Sp1 or Ets1 separately, combined siRNA targeting Sp1 and Ets1 did not further decrease the expression Prrx1 significantly, so it could be possible that the two pathway have a synergistic effect on Prrx1 expression Prrx1 mRNA level (Figure 4g). In addition, restoring the expression of Prrx1 partially recovered the expression of MMP2 and cyclinD1 in Sp1 or Ets1 siRNA-transfected LX-2 cells (Figure 4h and i). These data demonstrated that Sp1 and Ets1 are required for the PDGF-BB-mediated Prrx1 transcription activation.
Role of Prrx1 in PDGF-Induced Migration and Proliferation
PDGF has been reported to increase the chemotaxis and proliferation of HSCs during fibrosis.26 Next, we used the gain- and loss-of-function approaches in combination with various assays to decipher the role of Prrx1 as a modulator of PDGF-dependent processes. The silencing of Prrx1 in primary HSCs isolated from rat and LX-2 significantly reduced their rate of migration both basally and after PDGF-BB treatment (Figure 5a). In addition, Prrx1a obviously increased the migratory ability of LX-2 cells as compared with the cells transfected with Prrx1b plasmid or vectors (Figure 5b). Consecutively, we used the wound-healing assay to confirm the previous result. Consistent with the previous observations, Prrx1 knockdown attenuated the migration of HSCs in response to PDGF-BB stimulation (Figure 5c). The results indicated that Prrx1 might have a critical role in PDGF-dependent cell motility.
Prrx1 involved in PDGF-induced cell migration and knockdown of Prrx1 impaired cell adhesion to FN. (a and b) The migration rates of LX-2 cells and rat primary HSCs were measured after Prrx1 expression was silenced or enhanced in the presence or absence of PDGF-BB (15 ng/ml) by transwell migration assays. The number of migrated cells were counted in four random fields. Scale bars=100 μm. *P<0.05 siRNA vs NC; Prrx1 plasmid vs vector. (c) Representative images of the wound-healing assay with LX-2 cells. LX-2 cells were transfected with Prrx1 siRNA or NC in response to PDGF-BB stimulation. Cell migration was quantified as a percentage of wound-healed area. (d and e) Prrx1 expression was silenced in the presence or absence of PDGF-BB (15 ng/ml), representative images of adhesive cells stained with DAPI. The adhesion ability of HSCs was assessed by CCK-8. *P<0.05 vs NC or PDGF-BB.
Furthermore, to assess whether Prrx1 was involved in the regulation of PDGF-mediated proliferation, Prrx1 siRNA-treated HSC proliferation was assessed by CCK-8. Silencing the expression of Prrx1 did not affect the proliferation of HSCs (Supplementary Figure 2). These data demonstrated the vital role of Prrx1 in PDGF-mediated HSC migration and cell adhesion.
Reduced Prrx1 Expression Increases HSC Adhesiveness to Fibronectin
Cell adhesion and migration are critical pathological processes in liver fibrosis.27, 28, 29 To assess whether the defective migration of Prrx1 siRNA-transfected HSCs might result from altered cell adhesiveness, we performed a cell adhesion assay using fibronectin (FN) on HSCs. The knockdown of Prrx1 expression increased the adhesion of HSCs to fibronectin in the presence or absence of PDGF-BB (Figure 5d and e). Our results indicated that Prrx1 downregulation in HSCs significantly enhances their adhesiveness thereby impairing their ability of migration in response to PDGF-BB.
Prrx1 Regulates PDGF-Dependent Process by Modulation of cyclinD1 and MMPs
MMPs have a critical role in the degradation of ECM components; PDGF-BB induces MMP2- and MMP9-mediated migration of HSCs.26, 29, 30, 31 Therefore, we aspired to delineate whether Prrx1 regulated the downstream effectors in the PDGF-dependent physiological process. Here, we found that in LX-2 cells, Prrx1 siRNA prevented the PDGF-induced increase in the mRNA and protein levels of genes cyclinD1, MMP2, and MMP9 (Figure 6a–c). In addition, LX-2 cells transfected with Prrx1 plasmids and vector were then subjected to the treatment with PDGF-BB or vehicle, respectively. The results showed that the enforced expression of Prrx1 could upregulate MMP2 and MMP9 mRNA levels both at baseline and after PDGF-BB treatment. However, any distinct increase in the expressions of MMP2 and MMP9 was not found as compared with the LX-2 cells transfected with Prrx1 plasmids between vehicle and PDGF-BB stimulation group (Figure 6d). These data indicated that Prrx1 may be the key factor for PDGF-mediated MMP2/MMP9 upregulation. Next, we explored the relationship between Prrx1, Ets1, and Sp1, as all of them could regulate expression of the downsteam molecules MMP2. Through double immunofluorescence staining of Prrx1, Ets1, and Sp1 in LX-2, we could find that they were co-localized in the nucleus of LX-2 cells (Figure 6e). Besides, LX-2 cells were transfected with plasmid encoding Prrx1-Flag, then we investigated whether Prrx1 could work together with Ets1 or Sp1 by co-immunoprecipitation. The result showed that anti-Flag antibody co-immunoprecipitated complexes containing Sp1 and Ets1, and Prrx1 rabbit antibody identified the expression of Prrx1, which indicated Prrx1 could have interacted with Sp1 and Ets1 (Figure 6f and Supplementary Figure 3).
MMP2 and MMP9 were downstream targets of Prrx1 in PDGF-treated cells. (a and b) Prrx1 silencing in LX-2 cells and rat primary HSCs decreased the PDGF-induced upregulation of specific mRNAs. *P<0.05 vs NC or PDGF-BB. (c) LX-2 cells were transfected with Prrx1 siRNA in the presence or absence of PDGF-BB (15 ng/ml); western blot was used to examine the protein levels of Prrx1, MMP2, and cyclinD1. (d) LX-2 cells were transfected with Prrx1 or vector for 24 h, then stimulated with PDGF-BB (15 ng/ml) for an additional 48 h. The mRNA levels were tested by real-time PCR. *P<0.05 vs vector or PDGF+vector. (e) Immunofluorescence co-staining of Prrx1, Ets1, and Sp1 in LX-2 cells. (f) LX-2 cells were transfected with plasmids encoding Flag-Prrx1. After 48 h, cell lysates were prepared and used for co-immunoprecipitation.
Knockdown of Prrx1 Attenuates TAA-Induced Liver Fibrosis in Rat
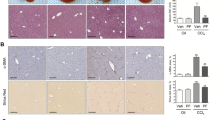
To confirm the role of Prrx1 on fibrogenesis in vivo, we silenced Prrx1 expression in a rat model of TAA-induced liver fibrosis. After tail-vein injection of AdshPrrx1, we observed that Prrx1 silencing resulted in reduced liver fibrosis by hematoxylin and eosin (H&E), Masson’s trichrome, α-SMA expression (Figure 7a and b and Supplementary Figure 4), and hydroxyproline contents (Figure 7c). In addition, the AdshPrrx1 administration significantly attenuated the liver damage as shown by decreased ALT and AST serum levels (Figure 7d). Furthermore, collagen1α1, α-SMA, and MMP2 levels were significantly reduced after AdshPrrx1 treatment (Figure 7e and f). Taken together, our results showed that Prrx1 silencing interferes with the development of TAA-induced liver fibrosis.
Knockdown of Prrx1 suppressed the TAA-induced hepatic fibrosis in rats. (a) AdshPrrx1 or AdshNC was injected into rats 6 weeks after treatment with TAA. Subsequently, after 2 weeks, the expression of COL1A1, α-SMA, MMP2, and Prrx1 in the fibrotic livers was analyzed by immunohistochemistry. HE and Masson’s trichrome staining were used to examine the pathological alterations and collagen deposition. (b) Semi-quantitative analysis of Masson’s trichrome staining in the fibrotic livers from AdshPrrx1- or AdshNC-treated rats (n=6 rats in each group). (c) Hydroxyproline level in livers from AdshPrrx1- or AdshNC-treated rats in the TAA-induced fibrotic model (n=6). (d) Serum biochemical parameters, ALT and AST, were analyzed from AdshPrrx1- or AdshNC-treated rats in the TAA-induced fibrotic model (n=4). (e and f) mRNA and protein levels of COL1A1, α-SMA, MMP2, and Prrx1 in the livers were detected by real-time PCR (n=5) and western blot (n=4), respectively. GAPDH was used as an endogenous control. *P<0.05, **P<0.01 by Student’s t-test.
Discussion
Previous studies have demonstrated that the serum level of PDGF-BB increased in patients with fibrosis of various etiologies and fibrotic livers in the mouse model.4, 32 PDGF is a principal growth factor that potently drives the proliferation and migration of HSCs.33 In recent decades, intensive research is focused on new therapies targeting the PDGF pathways; however, due to varied individual basis and low targeting efficiency, limited therapeutic advances have been made.34, 35 The current study, for the first time, demonstrates Prrx1 as a potential target in liver fibrosis that could regulate the PDGF-induced chemotaxis. Moreover, Prrx1 is stimulated by PDGF in a dose- and time-dependent manner in HSCs, which indicates the correlation between Prrx1 and PDGF stimulation. This phenomenon could also explain why Prrx1 is strongly expressed in HSCs in the fibrotic liver while being nearly undetectable in healthy liver tissue.
Prrx1, pair-related homeobox transcription factor, is upregulated 10–50-fold in the fibrotic liver and activated HSCs.36 Consistent with the previous studies, our results showed that Prrx1 expression was dramatically elevated in TAA- and CCl4-induced fibrosis. Despite the role of Prrx1 in HSC activation, we used complementary approaches including the gain- and loss-of-function to demonstrate the vital role of Prrx1 in the proliferation, motility, and cell adhesion of HSCs. We confirmed that the knockdown of Prrx1 impaired the motility of HSCs and increased cell adhesion in the presence or absence of PDGF. Conversely, the overexpression of Prrx1 enforced the migration of HSCs. This phenomenon could be further strengthened by the recent evidence that Prrx1 is an EMT inducer conferring migratory and invasive properties in pancreatic cancer and breast cancer cells.16, 18 The microarray data from another study showed that silencing Prrx1 in adult neural stem cells led to the upregulation of the cell adhesion molecules involved in the HSC-associated phenotypes.15 Furthermore, another report showed that in lung mesodermal cells, the expression of Prrx1 was essential for the aggregation of disorganized mesenchymal cells into an organized monolayer of vascular endothelium by cell-to-cell contact,37 which indicates the putative modulation of Prrx1 on cell adhesion. Collectively, these results indicated that Prrx1 could regulate the various cellular processes, such as synthesis of collagen, cell migration, and cell adhesion.
ERK and PI3K-Akt signaling pathways are known to be involved in PDGF-stimulated mitogenesis, migration, and chemotaxis.25 Our results indicated that ERK and PI3K-Akt cascades were vital for PDGF-induced activation of Prrx1 since the specific blockade of ERK or PI3K resulted in a significant reduction in the level of Prrx1 in the presence of PDGF. Furthermore, we demonstrated that the transcription factor Sp1, acting as a downstream signaling molecular of ERK, had a major role in the regulation of Prrx1 gene expression in HSCs, which is also supported by several lines of evidence. First, the inhibition of the ERK1/2 signaling pathway abolished the PDGF-BB-induced Sp1 and Prrx1 expressions. Second, the knockdown of Sp1 prevented the PDGF-BB-induced activation of the Prrx1 expression. Next, the stimulation with PDGF-BB enhanced the level of Sp1 protein similar to that of the Prrx1 level. Finally, the restoration of Prrx1 expression after silencing Sp1 recovered the expression of the downstream effectors, such as cyclinD1 and MMP2. Altogether, these findings, for the first time, revealed Sp1 as a downstream target of ERK1/2 in PDGF-stimulated HSC-regulated Prrx1 expression. This observation was in agreement with the previous study describing Sp1 activation as critical for PDGF-BB-mediated phenotypic modulation of smooth muscle.38 Similarly, we confirmed that PDGF increased the Prrx1 transcription via PI3K-mediated activation of p-Ets1; this was similar to the previous study showing Ets1 as a downstream molecule of PI3K regulated by PDGF.25 Compared with targeting Sp1 or Ets1 separately, LX-2 cells did not further decrease Prrx1 mRNA level when both Sp1 and Ets siRNAs are expressed together. It could be possible that the two pathways have a synergistic effect on Prrx1 expression. However, the precise mechanism underlying the activation of transcription factors Sp1 and Ets1 that regulate the expression of Prrx1 in HSCs necessitates further investigation.
The present study also provides an in vivo evidence that adenovirus-mediated knockdown of Prrx1 in TAA-induced liver injury model reduces the liver damage, inflammation, and development of liver fibrosis. Compared with the AdGFP injection, Prrx1 silencing significantly reduces the expression of HSC activation markers and attenuates the ECM deposition and liver function, which indicates that Prrx1 could attenuate liver fibrosis by deactivating the HSCs. This result was complementary to a previous study describing that adenovirus-mediated delivery of Prrx1a in mouse augmented collagen and the expression of α-SMA.20 Moreover, in agreement with the results that Prrx1 upregulated MMP2, MMP9, and cyclinD1 in vitro, the silencing of the gene leads to less-activated HSCs as presented in a fibrotic scar in vivo. This manifestation may be associated with the decrease in the motility and proliferation of HSCs and degradation of ECM. Liver fibrosis is always associated with enhanced expression of matrix protein and production of MMPs.26 The MMPs could proteolytically degrade ECM,39 as well as, contribute to cell adhesion and migration.27, 29, 31, 40 Therefore, Prrx1 mediated the upregulation of MMP2 and MMP9 expression that could decrease cell adhesion ability and promote the migration of HSCs. However, other underlying molecular and cellular mechanisms require further investigation.
In summary, we have identified Prrx1 as a novel valuable target in liver fibrosis, which had a major role in PDGF-induced migration and chemotaxis. Moreover, PDGF-BB via ERK-Sp1 and PI3K/Akt/Ets1 signaling increased Prrx1 expression in a dose- and time-dependent manner. Finally, our data also demonstrated that interference in Prrx1 expression could attenuate liver fibrosis development, partially by the repression of α-SMA, col1A1, and MMP2. Therefore, the novel regulatory mechanisms discovered about Prrx1 are speculated to provide an in-depth insight about PDGF signaling and a potential therapeutic target in liver fibrosis.
References
Schuppan D, Afdhal NH . Liver cirrhosis. Lancet 2008;371:838–851.
Hernandez-Gea V, Friedman SL . Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425–456.
Seki E, Schwabe RF . Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 2015;61:1066–1079.
Yoshida S, Ikenaga N, Liu SB et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014;147:1378–1392.
Hayes BJ, Riehle KJ, Shimizu-Albergine M et al. Activation of platelet-derived growth factor receptor alpha contributes to liver fibrosis. PLoS ONE 2014;9:e92925.
Wright JH, Johnson MM, Shimizu-Albergine M et al. Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: evidence for stromal induction of hepatocellular carcinoma. Int J Cancer 2014;134:778–788.
Soriano P . Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994;8:1888–1896.
Lindblom P, Gerhardt H, Liebner S et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 2003;17:1835–1840.
Kocabayoglu P, Lade A, Lee YA et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol 2015;65:1417–1417.
Grueneberg DA, Natesan S, Alexandre C et al. Human and drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science 1992;257:1089–1095.
Simon KJ, Grueneberg DA, Gilman M . Protein and DNA contact surfaces that mediate the selective action of the Phox1 homeodomain at the c-fos serum response element. Mol Cell Biol 1997;17:6653–6662.
ten Berge D, Brouwer A, Korving J et al. Prx1 and Prx2 are upstream regulators of sonic hedgehog and control cell proliferation during mandibular arch morphogenesis. Development 2001;128:2929–2938.
Dasouki M, Andrews B, Parimi P et al. Recurrent agnathia-otocephaly caused by DNA replication slippage in PRRX1. Am J Med Genet A 2013;161:803–808.
Martin JF, Olson EN . Identification of a prx1 limb enhancer. Genesis 2000;26:225–229.
Shimozaki K, Clemenson Jr GD, Gage FH . Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci 2013;33:4066–4075.
Reichert M, Takano S, von Burstin J et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev 2013;27:288–300.
McKean DM, Sisbarro L, Ilic D et al. FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. J Cell Biol 2003;161:393–402.
Ocana OH, Corcoles R, Fabra A et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012;22:709–724.
Fritz D, Stefanovic B . RNA-binding protein RBMS3 is expressed in activated hepatic stellate cells and liver fibrosis and increases expression of transcription factor Prx1. J Mol Biol 2007;371:585–595.
Jiang F, Stefanovic B . Homeobox gene Prx1 is expressed in activated hepatic stellate cells and transactivates collagen alpha1(I) promoter. Exp Biol Med (Maywood) 2008;233:286–296.
Lin J, Chen A . Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Invest 2008;88:529–540.
Zhang F, Kong D, Chen L et al. Peroxisome proliferator-activated receptor-gamma interrupts angiogenic signal transduction by transrepression of platelet-derived growth factor-beta receptor in hepatic stellate cells. J Cell Sci 2014;127:305–314.
Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology 2012;56:1870–1882.
Deaton RA, Gan Q, Owens GK . Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 2009;296:H1027–H1037.
Jinnin M, Ihn H, Asano Y et al. Platelet derived growth factor induced tenascin-C transcription is phosphoinositide 3-kinase/Akt-dependent and mediated by Ets family transcription factors. J Cell Physiol 2006;206:718–727.
Yang C, Zeisberg M, Mosterman B et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology 2003;124:147–159.
Atorrasagasti C, Aquino JB, Hofman L et al. SPARC downregulation attenuates the profibrogenic response of hepatic stellate cells induced by TGF-beta1 and PDGF. Am J Physiol Gastrointest Liver Physiol 2011;300:G739–G748.
Cao S, Yaqoob U, Das A et al. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest 2010;120:2379–2394.
Wang XM, Yu DM, McCaughan GW et al. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology 2005;42:935–945.
Naito T, Tanihata Y, Nishimura H et al. Expression of matrix metalloproteinase-9 associated with ets-1 proto-oncogene in rat tubulointerstitial cells. Nephrol Dial Transplant 2005;20:2333–2348.
Galli A, Svegliati-Baroni G, Ceni E et al. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology 2005;41:1074–1084.
Wilhelm A, Aldridge V, Haldar D et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut 2015;65:1175–1185.
Kong X, Horiguchi N, Mori M et al. Cytokines and STATs in liver fibrosis. Front Physiol 2012;3:69.
Mehal WZ, Schuppan D . Antifibrotic therapies in the liver. Semin Liver Dis 2015;35:184–198.
Popov Y, Schuppan D . Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology 2009;50:1294–1306.
Jiang F, Parsons CJ, Stefanovic B . Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol 2006;45:401–409.
Dettman RW, Steinhorn RH . Connecting the cells: vascular differentiation via homeobox genes and extracellular matrix in the distal lung. Circ Res 2004;94:1406–1407.
Azahri NS, Di Bartolo BA, Khachigian LM et al. Sp1, acetylated histone-3 and p300 regulate TRAIL transcription: mechanisms of PDGF-BB-mediated VSMC proliferation and migration. J Cell Biochem 2012;113:2597–2606.
Xu L, Hui AY, Albanis E et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 2005;54:142–151.
Wang H, Zhu Y, Zhao M et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2). PLoS ONE 2013;8:e70192.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81270507, No. 81572419).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
This study demonstrates that paired related homeobox protein 1 (Prrx1) acts as a critical downstream molecule in PDGF signaling pathway and plays a major role in the recruitment of PDGF-dependent hepatic stellate cells via modulation of matrix metalloproteinases 2 and 9. Additionally, knockdown of Prrx1 attenuates liver fibrosis in animal models.
Supplementary information
Rights and permissions
About this article
Cite this article
Gong, J., Han, J., He, J. et al. Paired related homeobox protein 1 regulates PDGF-induced chemotaxis of hepatic stellate cells in liver fibrosis. Lab Invest 97, 1020–1032 (2017). https://doi.org/10.1038/labinvest.2017.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.65
This article is cited by
-
Time and phenotype-dependent transcriptome analysis in AAV-TGFβ1 and Bleomycin-induced lung fibrosis models
Scientific Reports (2022)
-
The roles of ETS transcription factors in liver fibrosis
Human Cell (2022)
-
Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis
Scientific Reports (2021)
-
COL6A3 expression in adipose tissue cells is associated with levels of the homeobox transcription factor PRRX1
Scientific Reports (2020)
-
Transcriptional regulation of Hepatic Stellate Cell activation in NASH
Scientific Reports (2019)