Abstract
Crohn's disease (CD) and ulcerative colitis (UC) are idiopathic inflammatory conditions of the gut. Our goal was to investigate if invasive Escherichia coli strains were present in patients with inflammatory bowel disease (IBD). Bacterial strains were isolated from biopsy material obtained from normal controls, and patients with a clinical diagnosis of CD and UC. Invasive bacteria were characterized by gentamicin protection assay and biochemical profiling (Api-20E). Strains were characterized by induction of cytokine expression in epithelial and macrophage cell cultures, measurement of epithelial barrier function, and confocal microscopy. Of all invasive bacterial strains in CD 98.9% were identified as E. coli as opposed to 42.1% in UC and 2.1% in normal controls. Epithelial invasion in vitro was significantly higher for CD-associated E. coli (8.4%, ±5.5 of initial inoculum (I/O)) in comparison to UC (2.5%, ±0.4 I/O), but highest for strains from inflamed CD tissue (11.3%, ±4.3 I/O). Both, CD and UC E. coli strains induced high mean TNF-α expression in macrophage cell lines (2604.8 pg/105 cells, ±447.4; 2,402.6 pg/105 cells, ±476.3, respectively), but concentrations were significantly higher for isolates from inflamed CD tissue (3071.3 pg/105 cells, ±226.0). Invasive E. coli from IBD tissue induced similar concentrations of interleukin (IL)-8 in epithelial cell cultures, but strains from inflamed CD tissue induced significantly less epithelial IL-8 (674.1 pg/105 cells, ±58.0 vs 920.5 pg/105 cells, ±94.6). IBD-associated E. coli strains significantly decreased transepithelial resistance, induced disorganization of F-actin and displacement of ZO-1, and E-cadherin from the apical junctional complex (AJC). In comparison to normal controls and UC, E. coli are more prevalent in CD, are highly invasive, and do not encode for known effector proteins. E. coli strains from IBD patients regulate cytokine expression and epithelial barrier function, two pathological features of IBD.
Similar content being viewed by others
Main
Inflammatory bowel diseases (IBD), encompassing Crohn's disease (CD), and ulcerative colitis (UC) are chronically remitting intestinal inflammatory processes of unknown etiology.1 IBD predominantly affects young adults, and patients suffer from bloody diarrhea, abdominal pain, and weight loss. In a significant number of patients, inflammation is not limited to the intestine but affects extraintestinal sites, involving liver, eyes, and joints.2 Immunologically, IBD is characterized by a markedly increased cytokine expression from T lymphocytes, neutrophils, macrophages, and epithelial cells.3 Among others, significant cytokine induction has been observed for TNF-α4 and interleukin (IL)-8,5 two cytokines central to the pathogenesis of CD and UC. Increased colonic IL-8 concentration has been correlated with neutrophil infiltration of IBD tissue,6 whereas TNF-α is implicated in systemic manifestations of disease, like anorexia, weight loss, and depletion of whole-body protein and lipids.7 Additional pathophysiological change observed in IBD is elevated intestinal permeability that correlates with disease activity,8 and it has been utilized experimentally as a marker for relapsing disease.9 It has been speculated that the two essential components for the development of gastrointestinal inflammation in CD and UC are genetic predisposition of the host and environmental factors, although neither one alone is sufficient to induce chronic inflammation.
There is mounting evidence that the environmental factors implicated in the pathogenesis of IBD are bacteria and their components.10 Both, CD and UC primarily affect intestinal areas with high bacterial counts, and in CD, surgical diversion of fecal stream or treatment with bowel rest and total parental nutrition have been used therapeutically to control enteric inflammation.11 Further evidence implicating a role for enteric flora is that treatment with antibiotics and probiotics was found to be beneficial, and is now utilized for induction and maintenance of remission, respectively.12 In addition, immunological studies have shown that the majority of patients with IBD develop serological and T-cell response to their own enteric flora.13
Recently, a novel class of E. coli, termed adherent-invasive E. coli (AIEC), has been associated with CD.14 The representative lactose-fermenting strain LF82 efficiently invaded epithelial cell cultures in vitro and was able to survive in the intracellular compartment for prolonged period of time.15 The goal of the current study was to characterize invasive E. coli strains in an American population with CD, UC, or normal controls without IBD. Further, we sought to answer if invasive E. coli displays pathogenicity traits that have been identified in human IBD, namely invasion of epithelial cells, regulation of barrier function, and cytokine production.
Materials and methods
Biopsy Material
All studies were approved by the Emory Institutional Review Board. Patients with clinically confirmed IBD were recruited between July 2002 and August 2005 at a single center. With the exception of a single CD patient, research subjects did not have exposure to antibiotics 3 months before colonoscopy. Medications allowed during the study included azathioprine, prednisone, and mesalamine preparations. Patients receiving other medications for the treatment of IBD were excluded. Control patients constituted asymptomatic subjects with a normal colonoscopic examination and without evidence of inflammation or colonic polyps. After written consent, usually four biopsies were obtained during outpatient colonoscopy from 15 patients with CD (mean age 42.6 years, 60% women), 12 with UC (mean age 37.5 years, 75% women), and 12 normal control subjects (mean age 54 years, 54.3% women). For patients with a diagnosis of IBD, an attempt was made to gather tissue from macroscopically inflamed and non-inflamed appearing segments. Biopsies were immediately incubated in Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 100 μg/ml gentamicin (Invitrogen) for 1 h, washed three times in PBS and lysed in 1% Triton-X-100/PBS. Aliquots were cultured on MacConkey agar plates at 37°C overnight and lactose-fermenting colonies were enumerated and propagated in LB broth for 4 h at 37°C under aerobic conditions. Individual clones were stored in 50% glycerol at −80°C until further use.
Cell Culture
Caco-2 epithelial cells and J774A.1 macrophages (ATCC, Manassas, Virginia) were grown in DMEM (Invitrogen), gentamicin 50 μg/ml (Invitrogen), 10% FCS (Gemini, Calabasas, CA, USA) in a 5% CO2, 37°C atmosphere, and were used between passages 45–75 and 10–15, respectively. For invasion assays, epithelial cells and macrophages were seeded on collagen-coated 96-well plates. Caco-2 cells were allowed to differentiate for 5–7 days before infection.
Invasion Assay
Bacterial invasion was measured as described previously.16 Briefly, individual bacterial strains were grown aerobically in 2 ml LB broth at 37°C overnight. To minimize basolateral bacterial invasion, only differentiated Caco-2 epithelial cells were infected in triplicate at multiplicity of infection (MOI) 10:1 with an overnight culture. Following centrifugation at 500 g, 15 min, 37°C, infections were allowed to proceed for 3 h without antibiotics. Monolayers were washed in PBS four times and incubated for an additional hour in DMEM supplemented with 100 μg/ml gentamicin. Mean bactericidal concentration (MBC) for gentamicin was determined at 1 μg/ml and was used at 100-fold the MBC. Cell cultures were rinsed again in PBS after gentamicin killing and monolayer integrity was confirmed microscopically after the final wash. Epithelial cells were lysed in 1% Triton-X-100/PBS and serial dilutions were plated on LB plates. Colonies were counted the following day after 37°C incubation overnight. To determine the percentage of intracellular bacteria in relation to the initial inoculum (I/O), aliquots of individual overnight LB cultures were serially diluted, plated on LB agar plates, and enumerated. Significant epithelial invasion was determined by comparing pathogenic E. coli strains enteroinvasive E. coli strain E12860/0 (EIEC, kindly provided by MS Donnenberg), CD reference strain LF82 (kindly provided by A. Darfeuille-Michaud), to nonpathogenic, commensal strains EFC-1, and FN414. Invasion was considered significant if a minimum of ≥1% of I/O (t-test) could be recovered from the intracellular compartment. All bacterial strains used for this study are listed in Table 1.
Identification of Invasive Bacterial Strains
All bacterial strains considered to be invasive were grown on LB agar plates and a single colony was diluted in water. Aliquots of bacterial solutions were cultured in Api-20E test strips (Biomerieux, Durham, NC, USA) under aerobic and anaerobic conditions overnight according to the manufacturer's specification. Invasive bacteria classified as E. coli by the Api-20E system with likelihood values of ≥92.4% were considered for further analysis.
Multiplex PCR for Pathogenicity Genes in E. Coli Strains
E. coli strains determined to be invasive were subjected to multiplex PCR to screen for genes associated with pathogenicity as described previously. Briefly, invasive E. coli from our study and positive control strains, were boiled in PBS and amplified with primer combinations listed in Table 2. Pathogenic E. coli strains functioning as positive controls included Enterohemorrhagic E. coli O157:H7 (EHEC), EIEC E50851/0 (EIEC), Enteropathogenic E. coli 2348/69 (EPEC), Enterotoxigenic E. coli H10407, Enteroaggregative E. coli JM221 and 17-2, and Uropathogenic E. coli CFT073 and BF1072 (Table 1). PCR was performed with primer combinations for bacterial toxins (VT1, VT2, VT2e, ST1, ST2, LT-1, cnf1, cnf2, astA, cdt), adhesion molecules (fimH, eaeA, espBα, papC, afaB-afaC, sfaD-sfaE, bmaE/afaE8, pCVD432, bfp), EAF, and ipaH (Table 2). Bacterial DNA was amplified in 35 cycles with denaturing DNA at 94°C, annealing at 55°C, and extension at 68°C for 1 min each. PCR products were analyzed by agarose gel electrophoresis and stained with ethidium bromide.
Pulsed Field Gel Electrophoresis of Invasive E. Coli
Bacteria were grown on Trypticase soy agar plates with 5% sheep blood (TSA-SB; Becton Dickinson and Company, Sparks, MD, USA) at 37°C for 14–16 h. Pulsed field gel electrophoresis (PFGE) was performed using the PulseNet standardized protocol for E. coli O157:H7 as described by Ribot et al.22 Briefly, bacterial cells were suspended in cell suspension buffer (100 mM Tris, 100 mM EDTA, pH 8.0), and cell concentration adjusted to a turbidity reading of 0.48–0.52 (Dade Behring Microscan turbidity meter; Dade Behring Inc., Deerfield, IL, USA). A 400 μl aliquot of cell suspension was transferred to a sterile microcentrifuge tube containing 20 μl of proteinase K (20 mg/ml stock; Invitrogen, Carlsbad, CA, USA) followed by gentle mixing. Four hundred microliters of melted 1% SeaKem Gold agarose (FMC, Rockland, ME, USA) containing 1% SDS, made in standard 0.01 M Tris-EDTA buffer, were added to each cell suspension and mixed gently by pipetting up and down two to three times, immediately dispensing into the wells of PFGE plug molds (Bio-Rad, Hercules, CA, USA). The plugs were allowed to solidify for 5 to 10 min at room temperature. The solidified agarose plugs were transferred to a tube containing 5 ml of lysis buffer (50 mM Tris, 50 mM EDTA, 1% Sarkosyl (pH 8.0)) and 25 μl of proteinase K (20 mg/ml). Lysis was allowed to proceed at 54°C in a shaking water bath for 2 h. Plugs were washed two times with sterile water for 15 min each time and four times with sterile Tris-EDTA (10 mM Tris, 1 mM EDTA, pH8.0) for 15 min each time in a shaking water bath. Agarose-embedded DNA was cut (2.0 mm in thickness) and restricted with 50 U of XbaI and BlnI (Roche Molecular Biochemicals, Indianapolis, IN, USA) for 2 h at 37°C. The digested DNA plugs were loaded on the PFGE gel consisting of 1% SeaKem Gold agarose prepared in 0.5 × Tris-Borate-EDTA (TBE) buffer (Sigma, St Louis, MO, USA). The electrophoresis was carried out on a CHEF Mapper (Bio-Rad) with switch times of 2.16–54.17 s at 6 V/cm for 19 h and TBE buffer temperature at 14°C. Gels were stained using ethidium bromide (1 mg/ml) and destained with two deionized water washes. Gel images were obtained using a Gel Doc EQ imager (Bio-Rad) under UV transillumination. Analysis of PFGE patterns was performed using BioNumerics software package (Applied Maths, Sint-Martens-Latem, Belgium).
Cytokine Analysis
Caco-2 epithelial cells and J774A.1 macrophages were prepared as described above. Cell culture media were infected in triplicate with an aliquot of an overnight culture of control E. coli EFC-1, EPEC, and CD isolate LF82. Induction of cytokine expression was compared to strain 4F (CD, inflamed tissue), 13I (CD, normal tissue), and 30A (UC, normal tissue). Cells were infected at MOI 10:1 for 3 h, washed three times with PBS, and the growth medium was changed to DMEM supplemented with gentamicin 100 μg/ml, penicillin, and streptomycin. Cell supernatant was harvested after a total of 24 h and stored at −20°C until further use. Conditioned media were analyzed by ELISA (Invitrogen) in duplicate in three independent experiments for IL-8 and TNF-α from Caco-2 and J774A.1 cells, respectively, according the manufacturers specification. Data were expressed as mean with standard error (s.e.m.) per 1 × 105 cells.
Examination of Epithelial Barrier Function In Vitro
Well-differentiated Caco-2 cells were grown on polycarbonate membrane supports, pore size 0.4 μm (Costar, Corning, NY, USA). Subtracting filter resistance, epithelial monolayers were grown to an average transepithelial resistance (TER) of 327.9 Ωcm2, ±20.6 and infected with commensal E. coli strain EFC-1, pathogenic E2348/69 and representatives from patients with CD (LF82, 4F, 13I) and UC (30A) at MOI 10:1. E. coli isolates 4F, 13I, and 30A from our population were chosen for their high TNF-α-inducing capability. Changes in TER over time were determined hourly with an equilibrated Epithelial Voltohmmeter (World Precision Instruments, Berlin, Germany). Triplicate TER readings from three independent experiments were normalized, averaged, and expressed as percent change with standard error. The effect of bacterial isolates on adherens junctions (AJ) and tight junctions (TJ) was analyzed by confocal microscopy. Following infection for 4 h, cells were fixed in ice-cold 100% ethanol, and blocked with 5% goat serum in Hank's Balanced Salt Saline+ (HBSS) overnight. Epithelial monolayers were incubated with monoclonal mouse anti-ZO-1 (Zymed, San Francisco, CA, USA; dilution 1:1000) and mouse anti-E-cadherin (Becton Dickinson, Franklin Lakes, NJ, USA; dilution 1:300). Secondary antibodies used in our laboratory were goat anti-mouse Alexa546 and goat anti-rabbit Alexa647 (Molecular Probes, Carlsbad, CA, USA; dilution 1:1000). All monolayers were labeled with Alexa488-phalloidin to stain for F-actin (dilution 1:1000), nuclear stain Hoechst (dilution 1:10 000), and mounted in ProLong Gold (Invitrogen). Labeled slides were visualized using × 63 objective magnification in vertical (x/z) and horizontal plane (x/y) on an LSM510 confocal microscope (Zeiss, Jena, Germany).
Western Blot Analysis of Tight and Adherens Junctional Proteins
Polarized Caco-2 monolayers were propagated on permeable transwell polycarbonate membrane supports (Corning, NY, USA) and serum starved for 16 h before infection. Cells were infected with EFC-1, EPEC, LF82, 4F, 13I, or 30A at MOI of 10:1 for 4 h at 37°C with 5% CO2. Cells were lysed and scraped in 50 μl cell extraction buffer (Invitrogen) supplemented with complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA) and phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturers’ specifications. Protein concentration was determined using BCA protein assay kit (Pierce, Rockford, IL, USA). A 20–30 μg portion of cell extract was subjected to gel electrophoresis and transferred onto nitrocellulose membrane (Bio-rad). Membranes were blocked in 5% milk for 1 h at room temperature and labeled with primary antibodies at 4°C overnight. Primary antibodies for human TJ protein ZO-1 (Invitrogen), AJ protein E-cadherin (kindly provided by Dr Charles Parkos), and actin (Calbiochem, San Diego, CA, USA) were used at dilution 1:1000, 1:1000, and 1:500, respectively. Following labeling with primary antibodies, membranes were washed in TTBS and blotted with 1:20 000 HRP-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Labratories, West Grove, PA, USA). Membranes were developed with ECL substrate (Pierce, Rockford, IL, USA) and exposed to Kodak BioMax MR film (Eastman Kodak Company, Rochester, NY, USA). Film exposures from three independent experiments were subjected to UN-SCAN-IT densitometry analysis and data expressed as mean pixel with bars for s.e.m.
Statistical Analysis
For Figures 1, 2, 3 and 5 data were calculated as mean±s.e.m. The Student's t-test was used to determine statistical significance for invasion assays, ELISA, and measurements of TER. P-values ≤0.05 were considered statistically significant.
(a) Comparison of mean invasion indices with ±s.e.m. for E. coli isolates from patients with IBD. Differentiated Caco-2 cells in 96 wells were infected in triplicate for 3 h, culture medium changed, and supplemented with gentamicin. Aliquots of cell lysates and bacterial cultures were incubated on LB agar plates and bacterial invasion expressed as percentage of I/O. Mean invasion indices for E. coli from patients with CD were 8.4%, ±5.5 and UC 2.5%, ±0.4. Differences in mean invasion indices (Student's t-test) were considered significant comparing CD and UC (*P=1.22 × 10−7). (b) Mean invasion indices with ±s.e.m. for E. coli isolates from macroscopically normal and inflamed CD tissue. Mean invasion indices for E. coli isolated from inflamed tissue were higher (11.3%, ±4.3) in comparison with strains from normal appearing mucosa (7.3%, ±5.8). This did not reach statistical significance (P=5.34 × 10−2).
(a) E. coli strains from patients with CD and UC induce TNF-α expression in macrophage cultures in vitro. Individual invasive E. coli strains from patients with CD and UC were incubated with J774A.1 macrophages in duplicate for 3 h with an OD-adjusted aliquot of an overnight culture. Following 3 h infection, growth media were exchanged, now containing antibiotics, and TNF-α concentration in the supernatant was measured after 24 h. Data were expressed as mean TNF-α concentration per 1 × 105 cells with ±s.e.m. from three independent experiments (n=6) for isolates from CD (2604.8 pg/105cells, ±447.4), UC (2402.6 pg/105 cells, ±476.3), and negative control (752.3 pg/105cells, ±55.2). Differences in E. coli from CD and UC (Student's t-test) were not considered statistically significant (P=0.291). (b) E. coli from inflamed CD tissue induce high concentration of TNF-α in vitro. Separate analysis for induction of TNF-α expression in J774A.1 was performed for invasive E. coli strains propagated from inflamed and macroscopically normal appearing CD mucosa. E. coli from inflamed areas (3071.3 pg/105 cells, ±226.0) induced significantly more TNF-α in comparison to strains from normal mucosa (2476.5 pg/105 cells, ±447.9; *P=5.16 × 10−6). (c) E. coli strains from patients with IBD induce high TNF-α concentrations in macrophage cell cultures in vitro. J774A.1 macrophage cultures were infected at MOI 10:1 with E. coli control strains EFC-1, E2348/69, and LF82 and isolates 4F, 13I, and 30A from our IBD population for 3 h. TNF-α concentrations were determined after a total of 24 h by ELISA. Data are expressed as mean concentration per 105 cells with ±s.e.m. (n=6). Isolates LF82 (2978.3 pg/105 cells, ±346.3) and 13I (3771.9 pg/105 cells, ±41.9) from inflamed CD tissues induced significantly more TNF-α in comparison to E. coli EFC-1, E2348/69, 4F, and 30A.
(a) E. coli strains from patients with CD and UC induce IL-8 expression in Caco-2 cells in vitro. Individual E. coli strains from CD and UC patients were incubated in duplicate with Caco-2 monolayers. Epithelial IL-8 production was determined by ELISA after a total of 8 h from three independent experiments (n=6). Mean IL-8 concentrations were 850.8 pg/105 cells, ±107.3 for CD isolates and 1089.3 pg/105 cells, ±136.3 for UC E. coli. IL-8 induction was not statistically different for E. coli from CD and UC (P=0.0747). (b) Invasive E. coli from inflamed CD tissue induces less IL-8 expression in vitro. Mean IL-8 concentration in the supernatant of Caco-2 epithelial cells infected for 3 h with E. coli strains isolated from inflamed CD tissue was lower (674.1 pg/105 cells, ±58.0) in comparison with strains cultured from macroscopically normal appearing mucosa (920.5 pg/105 cells, ±94.6). This difference was statistically significant (*P=6.16 × 10−13). (c) Epithelial IL-8 expression in response to infection with individual E. coli strains. Supernatant of three independent experiments of Caco-2 cells infected with EFC-1, E2348/69, LF82, 4F, 13I, and 30A was analyzed by ELISA and data expressed as mean IL-8 concentration with ±s.e.m. (n=6). Control strains E2348/69 (1619.0 pg/105 cells, ±87.7) and LF82 (1719.4 pg/105 cells ±80.1) induced significantly higher IL-8 protein concentrations in comparison with nonpathogenic EFC-1 and isolates from our IBD population 4F, 13I, and 30A.
Results
Identification of Invasive Bacteria in CD, UC, and Normal Controls
Bacterial growth on MacConkey agar plates was detected in the form of individual lactose-fermenting and nonfermenting colonies. Colony density in biopsy material varied from patient to patient from a single to several hundred colonies. Bacterial growth following overnight incubation was detected in tissue from 12 of 15 patients with CD, 3 of 12 with UC, and 10 of 12 normal controls. Culture of IBD tissue samples resulted in bacterial growth regardless of whether biopsies were taken from macroscopically inflamed or normal appearing tissue. Following propagation under aerobic condition overnight, a total of 830 bacterial isolates were further analyzed in triplicate by an in vitro invasion assay with differentiated Caco-2 epithelial cells: 378 strains from CD, 111 from UC, and 341 from normal tissue (Table 3a). Significant invasion (≥1% I/O) was observed in 159 strains from all patients that underwent colonoscopy: 93 of 378 strains from patients with CD (24.6%), 19 of 111 from UC (17.1%), and 47 of 341 from normal control subjects (13.8%).
For identification purposes, 159 strains considered invasive were further subjected to biochemical testing by the Api-20E system. The majority of isolates were identified as E. coli (90.0%, Table 3b), followed by Enterobacter asburiae (4.5%), Klebsiella pneumoniae (1.8%), Hafnia alvei (0.9%), Buttiauxella agrestis (0.9%), and Escherichia hermannii (0.9%). Half of all patients with CD harbored invasive E. coli strains, whereas only two out of 15 subjects with UC were positive for invasive E. coli. Interestingly, invasive E. coli were only propagated from normal appearing UC tissue, but not from tissue samples taken from macroscopically inflamed areas.
Applying our experimental conditions, almost all invasive strains in CD were identified as belonging to the species E. coli (92 out of 93 strains, 98.9%; Table 3c). Invasive E. coli were less common in UC (42.1%). Out of 47 bacterial strains considered invasive, only a single E. coli (strain 150F, 2.1%) was identified in normal controls.
E. Coli Invasion Assay
We next determined invasion indices for all E. coli strains from patients with CD and UC, regardless of whether isolated from inflamed or normal appearing tissue. Mean invasion indices after 3 h of infection were significantly higher for E. coli isolates from CD patients (8.4%, ±5.5; Figure 1a) in comparison with strains from patients with UC (2.5%, ±0.4, p 1.22 × 10−7). Mean invasion index for the single invasive E. coli strain isolated from a normal control subject was 1.8%, ±1.4 (data not shown), which was significantly lower in comparison with E. coli isolates from patients with CD (P=0.026), but not UC (P=0.9).
In CD, invasive E. coli was cultured from both macroscopically normal and inflamed appearing tissue. In contrast to CD, we were only able to culture invasive E. coli from normal appearing UC tissues. Therefore, only invasion indices for E. coli strains from macroscopically inflamed and normal appearing CD tissue were further analyzed. Invasion indices for E. coli isolates from inflamed appearing mucosa were higher (11.3%, ±4.3; Figure 1b) in comparison to strains from non-inflamed areas (7.3%, ±5.8). However, this did not reach statistical significance (P=5.34 × 10−2).
Invasive E. Coli from IBD Tissue Induce Cytokine Expression In Vitro
LF82 is an AIEC representative strain isolated from a chronic CD lesion in the terminal ileum. In comparison to LPS, LF82 was previously found to induce 2.7-fold higher TNF-α concentrations in J774A.1 macrophage cultures after 24 h. We therefore sought to determine if Gram-negative isolates from our IBD population displayed a similar phenotype. E. coli cultures were incubated with J774A.1 macrophages at MOI 10:1 for 3 h, media supplemented with gentamicin, and TNF-α was measured in the cell supernatant after a total of 24 h. Mean TNF-α concentration for CD E. coli strains was 2604.8 pg/105 cells, ±447.4 (Figure 2a), which was similar for E. coli from patients with UC (2402.6 pg/105 cells, ±447.4, P=2.5 × 10−2), and was significantly increased from uninfected baseline (753.3 pg/105 cells, ±55.2). Analogous to invasion indices for CD E. coli from normal and inflamed tissue, we compared their ability to induce TNF-α expression in our macrophage culture system. E. coli from inflamed CD tissue induced significantly higher TNF-α secretion in the supernatant of J774A.1 cells compared with strains from normal appearing mucosa (Figure 2b; 3071.3 pg/105 cells, ±226.0 vs 2476.5 pg/105 cells, ±447.9; P=5.16 × 10−6).
Next, we focused on individual invasive bacterial isolates from our IBD population that induced very high TNF-α production in J774A.1 cultures. Isolates 30A from normal UC biopsy material, 4F and 13I from normal and inflamed CD tissue, respectively, were compared to the reference strain LF82, non-pathogenic EFC-1, and pathogenic EPEC strain E2348/69 in their ability to induce TNF-α. TNF-α expression in J774A.1 macrophage cultures was highest after infection with LF82 (Figure 2c; 2978.3 pg/105, ±346.3) and 13I (3991.9 pg/105, ±41.9, P=0.14). In comparison, infection with EFC-1 (955.0 pg/105 cells, ±73.7), E2348/69 (1302.8 pg/105, ±222.6), 4F (1890.8 pg/105 cells, ±119.9), 30A (1982.2 pg/105 cells, ±271.1), and invasive strain 150F from normal control tissue (1464.6 pg/105 cells, ±197.1, data not shown) led to significantly less TNF-α expression after 24 h in J774A.1 cultures. These results show that mucosal E. coli isolates from IBD patients induce significantly increased TNF-α expression, which is highest for isolates from the inflamed tissue.
Non-invasive and in particular invasive bacteria induce high IL-8 expression in epithelial cell cultures.23 We next subjected Caco-2 monolayer cultures to E. coli strains isolated from our IBD population and analyzed IL-8 expression in the supernatant of infected cell cultures. Monolayers were infected at MOI 10:1 in triplicate for 3 h without antibiotics, and changed to DMEM with antibiotics for the remainder of the experiment. Induction of IL-8 expression in epithelial cells increased from the baseline 477.3 pg/105 cells, ±4.4 (Figure 3a) to 850.8 pg/105 cells, ±107.3 for all CD strains and 1089.3 pg/105 cells, ±136.3 for isolates from UC tissue which did not reach statistical significance over CD strains (P=0.055). However, in contrast to increased TNF-α production in J774A.1 macrophage cultures, E. coli from inflamed as opposed to normal CD tissue induced significantly less IL-8 protein expression (Figure 3b; 672.2 pg/105 cells, ±58.5 vs 922.2 pg/105 cells, ±93.9). This difference in IL-8 expression for inflamed vs non-inflamed isolates was significant (P=3.95 × 10−13).
Analogous to experiments performed in macrophage cultures, we also compared IL-8 induction for individual IBD isolates from our population (4F, 13I, 30A) to E. coli reference strains EFC-1, E2348/69, and LF82. Highest concentrations of IL-8 expression in epithelial monolayer cultures were observed for E2348/69 (1619.0 pg/pg/105 cells, ±87.7) and LF82 (1,719.4 pg/105 cells ±80.1). None of our invasive IBD isolates (4F: 860.8 pg/105 cells, ±57.6; 13I: 606.4 pg/105 cells, ±53.5; 30A: 1183.6 pg/105 cells, ±52.4) or E. coli strain 150F from a control subject (389.3pg/105 cells, ±70.7; data not shown) was capable of inducing comparable level of IL-8 in the supernatant of infected Caco-2 monolayer cultures. These results indicate that invasive E. coli isolates from our population are either capable of weakly inducing or suppressing IL-8 expression in Caco-2 monolayer cultures.
Examination of Genetic Similarity among Invasive E. Coli Strains from IBD Patients
Following phenotypic characterization by invasion assay and cytokine expression, we determined genetic relatedness of IBD E. coli isolates by PFGE (Figure 4). To increase accuracy of genetic analysis, whole bacterial DNA isolated from EFC-1, LF82, 4F, 13I, and 30A was subjected to digestion with restriction enzymes XbaI and BlnI and gel electrophoresis. The highest degree of similarity was determined for isolates 4F and 13I (64.71%) from our CD patient population. However, this did not reach statistical significance. Genetic similarities were even lower for all other E. coli strains tested, including LF82 and EFC-1. Therefore, E. coli from CD and UC patients appear to share certain phenotypic characteristics like epithelial invasion and cytokine induction, but are not genetically related.
E. coli PGFE. Agarose-embedded bacterial DNA from strains EFC-1, LF82, 4F, 13I, and 30A was digested with XbaI and BlnI, and electrophoresis carried out with switch times of 2.16 to 54.17 s at 6 V/cm. Gels were stained using ethidium bromide and images obtained with a Gel Doc EQ imager under UV transillumination. Analysis of PFGE patterns was performed using BioNumerics software package. No significant similarity was determined for IBD isolates from patients with either CD or UC.
Analysis for Effector Proteins in Invasive E. Coli from IBD Tissue
Amplification of bacterial DNA with all primer combinations for bacterial toxins and adhesion molecules resulted in a PCR product of correct size only in positive control strains. Unlike control strains, none of the E. coli isolates from our IBD population yielded a positive result for any of the tested primer combinations, including afaB-afaC and fimH (data not shown). These findings indicate that our E. coli strains might harbor novel genes associated with bacterial adhesion and invasion.
Regulation of Barrier Function in Response to Infection with IBD E. Coli
Increased intestinal permeability is a feature of patients with IBD and their relatives, in particular CD.24 We therefore sought to determine if our IBD bacterial isolates regulate protein members of the apical junctional complex (AJC) in differentiated epithelial cell culture in vitro. Epithelial monolayer was grown to an average TER of 327.9 Ωcm2, ±20.6 and infected with commensal E. coli strain EFC-1, E2348/69, and representatives from patients with CD (LF82, 4F, 13I) and UC (30A) at MOI 10:1. In comparison to uninfected negative control and EFC-1 infected monolayers, E2348/69 and all IBD strains tested (LF82, 4F, 13I, 30A) induced comparable and significant decrease in TER over time regardless of whether they were isolated from CD or UC tissue samples (Figure 5). These differences in TER became apparent at 4 h, were significant at 5 h, and progressed until the end of the experiment at 11 h. Our results indicate that E. coli strains from patients with IBD regulate barrier function.
Invasive E. coli from CD and UC reduce epithelial barrier function. Epithelial monolayers cultures were grown to an average TER of 327.9 Ωcm2, ±20.6 and were infected in triplicate with commensal, nonpathogenic EFC-1, E2348/69, and representative E. coli strains from patients with CD (LF82, 4F, 13I) and UC (30A) at MOI 10. TER was measured hourly and followed up to 11 h, data from three independent experiments (n=9) normalized and expressed as percent change over baseline with standard error. As opposed to untreated negative control monolayer and infection with EFC-1, treatment with E2348/69, LF82, 4F, 13I, and 30A led to reduction of TER evident at 4 h and significant (*) at 5 h which progressed until the end of the experiment at 11 h.
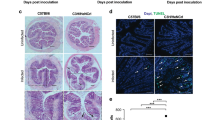
For confocal microscopy, Caco-2 monolayers grown on membrane supports were infected at MOI 10:1 for 4 h and analyzed by confocal microscopy. In comparison to uninfected control slides, nonpathogenic commensal strain EFC-1 induced minimal reorganization of F-actin, but not displacement of TJ protein ZO-1 or AJ protein E-cadherin (Figure 6). However, E2348/69 (Figure 6, inset) and LF82, isolated from inflamed CD tissue, reorganized F-actin, and displaced ZO-1 and E-cadherin from the AJC. Similar changes were observed for strain 13I and 30A from our study population, isolated from inflamed CD and normal UC tissue, respectively. Displacement of ZO-1 and E-cadherin, and the disorganization of F-actin were less severe after infection with an E. coli isolates from non-inflamed CD (4F). Regardless of the Gram-negative strains used to infect Caco-2 cells, all induced significant apical aggregation of F-actin in comparison to negative control cell cultures (Figure 6, x/z). Despite disorganization of F-actin and displacement of ZO-1, and E-cadherin in cell cultures infected with E. coli, columnar cell shape was maintained as determined by nuclear stain and F-actin organization (data not shown). Additionally, infection with any E. coli strains used in this study did not result in changes of total cellular concentrations for ZO-1, E-cadherin, or β-actin as determined by Western blot analysis and densitometry (Supplementary data). Invasive IBD E. coli strains induce displacement of AJC family of proteins which results in decreased epithelial barrier function in vitro.
Examination of Caco-2 monolayer cultures infected with E. coli by confocal microscopy. Polarized Caco-2 cells were grown on membrane supports and infected for 4 h with nonpathogenic commensal strain EFC-1, positive control E2348/69, reference strain LF82, and invasive E. coli from our population: 4F (inactive CD), 13I (inflamed CD), 30A (normal UC). Monolayers were fixed, stained, and examined by confocal microscopy. Infection with LF82, 13I, and 30A resulted in significant displacement of ZO-1 (red), E-cadherin (red), and F-actin (green). Displacement of AJC proteins was less severe for strain 4F isolated from normal CD tissue and minimal for commensal EFC-1. Infection with all E. coli isolates resulted in apical accumulation of F-actin (x/z; red: E-cadherin, green: F-actin). Monolayer integrity was not affected in negative controls or experimental samples infected with E. coli (data not shown).
Discussion
This study investigated whether invasive E. coli are present in an American population of normal controls and patients with IBD. Our findings show that invasive E. coli are predominantly associated with CD and to a lesser extent with UC, but not normal control subjects. Interestingly, almost all invasive bacterial strains isolated from CD patients were identified as E. coli regardless of tissue disease activity. Applying our methods of bacterial isolation, we were unable to isolate invasive E. coli strains from inflamed UC tissue. Invasion indices for CD E. coli were significantly higher in comparison to those from UC and even higher for strains from inflamed CD tissue. We found that E. coli from both CD and UC induced significant TNF-α expression in macrophage supernatant comparable to the reference strain LF82 from a patient with CD. Strains from both IBD populations induced IL-8 in epithelial cells, but E. coli from inflamed CD tissue induced significantly less. In addition, invasive E. coli strains from our IBD population decreased epithelial barrier function through displacement of the key AJC proteins ZO-1 and E-cadherin. Induction of IL-8 and TNF-α expression5 and decrease in barrier function25, 26 have been described as pathophysiological features of IBD.
E. Coli in IBD
E. coli is the predominant facultative aerobic inhabitant of human intestine and part of normal enteric flora. There is mounting evidence that E. coli is more prevalent in patients with IBD in comparison to normal control subjects, particularly in CD tissue.27 Initial studies, determining bacterial colonization in resected ileal and colonic surgical specimens, identified significantly increased E. coli colony counts in CD, but not tissue from patients with UC or normal controls.28 Recently, higher numbers of E. coli were also identified in neoterminal ileum of CD, especially in early recurrent disease when compared with normal control tissue.29 Alternatively, PCR-based methods detected E. coli 16S rRNA and DNA more frequently in CD biopsy material than control tissue30, 31 In comparison to tissue infected with other enteric pathogens, immunohistochemistry identified E. coli antigens in biopsy material from 10 out of 16 CD patients, especially in ulcerations, lamina propria, and along fissures.32 However, several studies found IBD-associated E. coli with both CD and UC. Culture-independent analysis of bacterial ribosomal RNA revealed significantly higher prevalence of E. coli in IBD in comparison with normal control subjects.33, 34 This was confirmed with a culture-dependent method, as epithelium-associated E. coli was propagated from CD and UC tissue, but not from normal controls.35
An increasing number of pathogenicity features are being identified in E. coli from patients with CD36, 37, 38 and in animal models, one of them being bacterial invasion. LF82 is an invasive E. coli isolated from an ileal biopsy that efficiently adheres to and invades epithelial cells in vitro,14 and has been used in this and other studies as a reference strain. The association of CD with invasive E. coli was confirmed by Martin et al39 as 29% of CD patients were infected with intramucosal E. coli in comparison with 9% of control subjects. Bacterial invasion was verified by infecting epithelial cell cultures in vitro. Similar results were described for granulomatous colitis, an IBD equivalent in boxer dogs, identifying adherent, invasive, and replicating E. coli within cultured epithelial cells.40 A role of invasive bacteria in CD is indirectly supported by the fact that E. coli were more frequently identified in serosa and mesenteric lymph nodes from CD surgical specimen than healthy control tissue41, 42 Our findings reported here support these studies and support a role for invasive E. coli in the pathogenesis of CD.
Our findings are in contrast to other studies investigating quantitative changes of the intestinal flora in IBD.43 Swidsinski et al44 described E. coli adherent to, but not invading the epithelial layer, even in inflamed mucosa. In that particular study, invasive bacteria were rare and identified predominantly as Bacteroides fragilis and Eubacterium rectale populations. These differences in results might be explained by alternative experimental approaches utilized. We speculate that in vivo, E. coli strains tightly adhere to epithelial cells and are prevented from invading tissue by host factors, including the mucosal immune system, peristalsis, and mucus production. However, propagation ex vivo unmasks bacterial pathogenicity traits and E. coli become invasive in a system devoid of additional host defense mechanisms.
Invasive E. Coli Induce TNF-α Expression
In comparison with nonpathogenic commensal strains and LPS, we show that E. coli isolates from our IBD population induced significantly higher TNF-α expression in macrophage cell cultures in vitro. Maximum expression of TNF-α in J774.A1 cultures in response to our isolates was comparable to concentrations induced by reference E. coli strain LF82 propagated from a chronic small bowel CD lesion.15 Higher TNF-α stimulatory activity of isolates from CD and UC patients might be due to quantitative and qualitative differences in LPS, flagella, or due to a specific bacterial effector gene or gene products. LPS and flagellin mediate activation of the innate immune response through Toll-like receptor-4 and -5 (TLR), respectively, leading to marked upregulation of TNF-α.45 TLR-mediated induction of cytokine expression in IBD appears to be a strong candidate pathway as stimulation of macrophages collected from IBD patients with TLR-2 agonists zymosan and lipoteichoic acid induces TNF-α in vitro.46 This study also identified an increased expression of TLR-2 on macrophages isolated from patients with active CD in comparison to cells from patients in remission or control subjects. An additional candidate receptor possibly mediating increased TNF-α expression is TLR-4, which was upregulated on macrophages from inflamed CD and UC tissue.47 This requires further clarification, considering the central role of TNF-α in the pathogenesis and therapy of patients with IBD.48, 49, 50, 51
Invasive E. Coli Regulate IL-8 Activation
Pathogenic bacteria, and in particular strains that penetrate the epithelial barrier, induce high expression of pro-inflammatory cytokines, including TNF-α and IL-8.23 Similar to the reference strain LF82, invasive E. coli strains from our patient population with CD and UC induced high expression of epithelial IL-8. Maximum IL-8 expression was not different among the disease groups, but significantly decreased in epithelial cultures infected with E. coli from inflamed tissue. This novel finding was unexpected, considering a stimulatory effect of IBD-associated E. coli from our population on TNF-α expression in macrophage cultures as outlined above.
Suppression of IL-8 is a common feature among Gram-negative pathogenic and nonpathogenic bacteria, and has been described for Salmonella spp. and Yesinia spp. Following epithelial cell invasion, Salmonella enterica serovar typhimurium was found to inhibit NF-κB-dependent gene expression, including IL-8.52 The effector proteins responsible for this effect were identified as SspH1 and SptP. Similarly, nonpathogenic Salmonella typhimurium Pho1Pc and S. pullorum express AvrA, a bacterial protein that prevents TNF-α-induced IL-8 transcription.53 YopJ, a bacterial effector similar to AvrA, blocked transcription of IL-8 in bronchial epithelial cells.54 In addition to IL-8, YopJ also reduced expression of RANTES and ICAM-1 in the same in vitro system. Even though IL-8 is increased in CD tissue,55 we speculate that our invasive, activated E. coli isolated from inflamed areas express a single or multiple effector molecules that prevent maximal expression of IL-8 by epithelial cells.
Regulation of Barrier Function
Our study clearly showed for the first time that E. coli isolated from patients with IBD decrease TER and induce disorganization of F-actin, and displacement of ZO-1, and E-cadherin. Previous studies have identified a twofold increase of intestinal permeability in patients with CD and their relatives.56 This has been attributed to a decrease in mRNA and protein concentrations of E-cadherin25 and ZO-157 among other molecules. Besides, cytokines such as TNF-α,58 α-hemolysin from CD E. coli59 has been implicated in the regulation of barrier function. It is therefore conceivable that our invasive E. coli elicit, at least in part, a direct and indirect effect on barrier function through upregulation of TNF-α.
In conclusion, our present findings associate invasive E. coli with IBD, in particular CD, and describe novel pathogenicity features of these bacteria including regulation of cytokine expression and barrier function. Disturbance of immune balance and barrier function by invasive bacteria might account for some of the pathophysiological changes and intestinal inflammation observed in IBD. Future studies will include serological and genetic analyses of our invasive E. coli and dissection of TNF-α regulatory pathways through TLR.
References
Fiocchi C . Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205.
Hendrickson BA, Gokhale R, Cho JH . Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15:79–94.
Rogler G, Andus T . Cytokines in inflammatory bowel disease. World J Surg 1998;22:382–389.
Akazawa A, Sakaida I, Higaki S, et al. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol 2002;37:345–353.
McCormack G, Moriarty D, O’Donoghue DP, et al. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res 2001;50:491–495.
Mitsuyama K, Toyonaga A, Sasaki E, et al. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol 1994;96:432–436.
Tracey KJ, Cerami A . Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med 1994;45:491–503.
Miki K, Moore DJ, Butler RN, et al. The sugar permeability test reflects disease activity in children and adolescents with inflammatory bowel disease. J Pediatr 1998;133:750–754.
Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15–22.
D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–267.
Seo M, Okada M, Yao T, et al. The role of total parenteral nutrition in the management of patients with acute attacks of inflammatory bowel disease. J Clin Gastroenterol 1999;29:270–275.
Sartor RB . Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620–1633.
Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–455.
Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun 1999;67:4499–4509.
Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun 2001;69:5529–5537.
Donnenberg MS, Donohue-Rolfe A, Keusch GT . A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett 1990;57:83–86.
Mobley HL, Green DM, Trifillis AL, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 1990;58:1281–1289.
Taylor DN, Echeverria P, Sethabutr O, et al. Clinical and microbiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J Clin Microbiol 1988;26:1362–1366.
Tzipori S, Karch H, Wachsmuth KI, et al. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun 1987;55:3117–3125.
Donnenberg MS, Donohue-Rolfe A, Keusch GT . Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis 1989;160:452–459.
Nataro JP, Deng Y, Cookson S, et al. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis 1995;171:465–468.
Ribot EM, Fair MA, Gautom R, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 2006;3:59–67.
Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995;95:55–65.
Katz KD, Hollander D, Vadheim CM, et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology 1989;97:927–931.
Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 2001;281:G216–G228.
Blair SA, Kane SV, Clayburgh DR, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006;86:191–201.
Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 2004;127:412–421.
Keighley MR, Arabi Y, Dimock F, et al. Influence of inflammatory bowel disease on intestinal microflora. Gut 1978;19:1099–1104.
Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol 2002;97:939–946.
Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, et al. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 2006;12:1136–1145.
Ryan P, Kelly RG, Lee G, et al. Bacterial DNA within granulomas of patients with Crohn's disease—detection by laser capture microdissection and PCR. Am J Gastroenterol 2004;99:1539–1543.
Cartun RW, Van Kruiningen HJ, Pedersen CA, et al. An immunocytochemical search for infectious agents in Crohn's disease. Mod Pathol 1993;6:212–219.
Kotlowski R, Bernstein CN, Sepehri S, et al. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 2006;13:675–683.
Fujita H, Eishi Y, Ishige I, et al. Quantitative analysis of bacterial DNA from Mycobacteria spp. Bacteroides vulgatus, and Escherichia coli in tissue samples from patients with inflammatory bowel diseases. J Gastroenterol 2002;37:509–516.
Mylonaki M, Rayment NB, Rampton DS, et al. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:481–487.
Barnich N, Boudeau J, Claret L, et al. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol Microbiol 2003;48:781–794.
Barnich N, Bringer MA, Claret L, et al. Involvement of lipoprotein NlpI in the virulence of adherent invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect Immun 2004;72:2484–2493.
Bringer MA, Barnich N, Glasser AL, et al. HtrA stress protein is involved in intramacrophagic replication of adherent and invasive Escherichia coli strain LF82 isolated from a patient with Crohn's disease. Infect Immun 2005;73:712–721.
Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 2004;127:80–93.
Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun 2006;74:4778–4792.
Laffineur G, Lescut D, Vincent P, et al. Bacterial translocation in Crohn's disease. Gastroenterol Clin Biol 1992;16:777–781.
Ambrose NS, Johnson M, Burdon DW, et al. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn's disease surgery. Br J Surg 1984;71:623–625.
Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54.
Swidsinski A, Weber J, Loening-Baucke V, et al. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 2005;43:3380–3389.
Abreu MT, Fukata M, Arditi M . TLR signaling in the gut in health and disease. J Immunol 2005;174:4453–4460.
Canto E, Ricart E, Monfort D, et al. TNF alpha production to TLR2 ligands in active IBD patients. Clin Immunol 2006;119:156–165.
Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 2002;122:1987–2000.
Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–1811.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–2476.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359:1541–1549.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 1997;337:1029–1035.
Haraga A, Miller SI . A Salmonella enterica serovar typhimurium translocated leucine-rich repeat effector protein inhibits NF-kappa B-dependent gene expression. Infect Immun 2003;71:4052–4058.
Collier-Hyams LS, Zeng H, Sun J, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol 2002;169:2846–2850.
Zhou L, Tan A, Hershenson MB . Yersinia YopJ inhibits pro-inflammatory molecule expression in human bronchial epithelial cells. Respir Physiol Neurobiol 2004;140:89–97.
Daig R, Andus T, Aschenbrenner E, et al. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 1996;38:216–222.
Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 1986;105:883–885.
Kucharzik T, Walsh SV, Chen J, et al. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 2001;159:2001–2009.
Ye D, Ma I, Ma TY . Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 2006;290:G496–G504.
Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 1998;115:1405–1413.
Acknowledgements
We are indebted to Dr A Darfeuille-Michaud (LF82) and Dr MS Donnenberg (EIEC E12860/0, EPEC E2348/69, EHEC EDL-933, ETEC H10407), for providing reference E. coli strains used in our studies. JMAK is supported by Public Health Service Grant DK0628990-02, DK64399, and R24EK064399-04, AN by DK55679 and CCFA, and SVS by DK064711-03.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website (http://www.laboratoryinvestigation.org)
Supplementary information
Rights and permissions
About this article
Cite this article
Sasaki, M., Sitaraman, S., Babbin, B. et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest 87, 1042–1054 (2007). https://doi.org/10.1038/labinvest.3700661
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700661
Keywords
This article is cited by
-
Characterization of mucosa-associated Escherichia coli strains isolated from Crohn’s disease patients in Brazil
BMC Microbiology (2020)
-
Study of a classification algorithm for AIEC identification in geographically distinct E. coli strains
Scientific Reports (2020)
-
High carriage of adherent invasive E. coli in wildlife and healthy individuals
Gut Pathogens (2018)
-
AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation
Scientific Reports (2018)
-
Comparative genomics reveals new single-nucleotide polymorphisms that can assist in identification of adherent-invasive Escherichia coli
Scientific Reports (2018)