Abstract
Neonatal hypothermia is an important risk factor for mortality and morbidity, and is common even in temperate climates. We conducted a systematic review to determine whether plastic coverings, used immediately following delivery, were effective in reducing the incidence of mortality, hypothermia and morbidity. A total of 26 studies (2271 preterm and 1003 term neonates) were included. Meta-analyses were conducted as appropriate. Plastic wraps were associated with a reduction in hypothermia in preterm (⩽29 weeks; risk ratio (RR)=0.57; 95% confidence interval (CI) 0.46 to 0.71) and term neonates (RR=0.76; 95% CI 0.60 to 0.96). No significant reduction in neonatal mortality or morbidity was found; however, the studies were underpowered for these outcomes. For neonates, especially preterm, plastic wraps combined with other environmental heat sources are effective in reducing hypothermia during stabilization and transfer within hospital. Further research is needed to quantify the effects on mortality or morbidity, and investigate the use of plastic coverings outside hospital settings or without additional heat sources.
Similar content being viewed by others
Introduction
Almost 3 million newborns die within the first month of life, with 1 million deaths occurring within 24 h of birth.1 Rates of neonatal mortality are appreciably higher in low- and middle-income countries, especially outside of hospitals.2 Although child mortality has been almost halved since the 1990 baseline of the Millennium Development Goals, progress in reducing neonatal mortality has been much slower; 44% of all child deaths now occur in the neonatal period.2
Direct complications of preterm birth account for over one-third of neonatal deaths with hypothermia being an important contributing factor to mortality among preterm neonates.3, 4 There is a high prevalence of hypothermia globally, with in-hospital estimates of between 32 and 85%, and in homes between 11 and 92%.5 Other estimates show mortality among hypothermic neonates to be twice that for term and 30 times that for preterm normothermic infants.6 Hypothermia also increases rates of infection, respiratory complications, acidosis and coagulation defects among neonates.7
Newborns are at risk for hypothermia when they are delivered from the warm intrauterine environment. They are particularly at risk if they are not immediately dried, as in addition to convective heat loss, they also lose heat via the evaporation of amniotic fluid. Preterm neonates are particularly vulnerable to heat loss given their thin epidermis, large surface area-to-weight ratio, lack of fat stores for thermogenesis and immature thermoregulatory mechanisms, such as a lack of vasomotor control.8
Thermal care practices have long been a cornerstone of neonatal care. Temperature maintenance for newborns has evolved over the years from the use of incubators in the 1800s to various combinations of technologies including radiant warmers, heated mattresses and family-led practices such as delayed bathing, wrapping the neonate, hats and skin-to-skin care.9, 10 As many of these practices were standard care before the use of randomized controlled trials (RCTs), there is limited rigorous evidence for the temperature, morbidity or mortality effects of using one strategy in isolation.9, 11 Although the advent of Kangaroo Mother Care has shown large reductions in mortality and hypothermia,12, 13 only one study to date has examined this method for stabilization immediately following delivery, the most vulnerable period for hypothermia. Despite numerous thermal care interventions, there continue to be high rates of hypothermia in low- to middle-income countries in hospitals as well as in homes. There is need for a low-cost, easy-to-use, accessible solution for temperature stabilization immediately following delivery.
Plastic covering the torso and extremities of neonates is recognized as a low-cost means to prevent hypothermia by reducing both conductive and evaporative heat loss.14, 15 A recent Cochrane review of interventions to prevent hypothermia in preterm neonates suggested that plastic bags reduced heat losses by 0.7 °C in neonates <28 weeks, with a 44% reduction in hypothermia observed. No heat loss reduction was observed in neonates 28 to 31 weeks, and no mortality reduction was seen for any gestational age.10 Since this review, further studies have been published, including from low- to middle-income countries, as well as studies on term neonates. As there is no conclusive evidence or guidelines on the use of plastic coverings,16 the objective of this review is to assess the impact of plastic coverings on neonatal mortality and morbidity. We also quantify the risk of hyperthermia.17 Our results are presented according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18
Methods
Searches
Systematic searches were performed on PubMed, EMBASE, Ovid, Cochrane Library, LILACS, African Index Medicus, and EMRO databases (last search 8 January 2015). The search strategy used a combination of MESH and free text terms including newborn and wrap terms combined with temperature- or hypothermia-related outcome terms (Supplementary Table). No date or language restrictions were put on the search. The full texts were reviewed for all potentially relevant studies. Hand searches of their reference lists were also conducted. Studies meeting inclusion criteria were abstracted onto Excel.
Inclusion/exclusion criteria
We used a PICO approach (population, intervention, comparison, outcome) to define the studies of interest. The ‘population’ included preterm and term neonates, born vaginally or by cesarean section, in a hospital or community-based setting. The ‘intervention’ was wrapping the neonate’s body in a plastic wrap or bag immediately following birth. The wrap/bag was then kept on during transport to the nursery (for term neonates) or the neonatal intensive care unit for preterm neonates. The plastic was removed after the neonate had been stabilized. Although the interventions followed a similar form, flexibility was given for the duration of use (either based on specific time limits or when the neonate reached normothermia), whether temperature was measured at the axilla or rectum, temperature cutoffs used to define hypo- and hyperthermia, as well as for the type of plastic device used for wrapping. The plastic material used in the studies ranged from Saranwrap to grocery store plastic bags as well as manufactured plastic sheets. The ‘comparison’ group consisted of neonates receiving only conventional thermal care, including hats, incubators, radiant warmers, warmed rooms and delayed bathing. The ‘outcomes’ of interest were mortality or morbidity, including hypothermia, hyperthermia, temperature change and preterm birth complications, for example, intraventricular hemorrhage, necrotizing enterocolitis and bronchopulmonary dysplasia.
We included both observational studies and RCTs that fulfilled the inclusion criteria. We excluded studies not fulfilling these criteria, duplicate reports and those published in a language other than English, French or Spanish. Studies from any country were included. We did not exclude studies based on sample size, as all studies contributed to a larger pooled estimate.
Data synthesis
We assessed the quality of the included studies using adapted GRADE criteria.19 Stringent criteria were used in the decision of pooling studies for meta-analysis. First, studies were divided based on the gestational age of the newborns (or birth weight if gestation was not provided); very preterm (⩽29 weeks or ⩽1 kg), mixed preterm (24 to 37 weeks) and term (⩾37 weeks). These groupings were made as we expected a differential increase in temperature from plastic coverings based on gestational age, with the most preterm infants having the greatest and term infants having the least change.20, 21, 22, 23 Second, we assessed the comparability of the study designs, control and intervention group definitions as well as the outcomes recorded. Meta-analyses were performed where data were available from two or more studies with similar designs and outcomes. Where less than two studies were found or the studies were not sufficiently comparable, the results were presented in tabular form and discussed in the text. Summary estimates are presented as risk ratios (RRs) with corresponding 95% confidence intervals (CIs). Temperature change is measured in °C with corresponding s.d.’s. We evaluated evidence of statistical heterogeneity using the I2 statistic, with >50% considered to represent substantial heterogeneity. We further considered the magnitude and direction of the intervention effects and the P-value from χ2.19 When substantial heterogeneity was present, causes were investigated using sensitivity analyses and use of random effects models. All analyses were performed in Stata 11 (StataCorp, College Station, TX, USA).24
Results
Our searches identified 297 unique articles from the database searches, with an additional six studies found from examining the reference sections of included papers. We retrieved and reviewed the full text of 57 articles. A total of 26 articles were included in the analysis (Figure 1; Table 1). Of the included studies, the majority (22) only involved preterm neonates; 18 included preterm neonates (<1.5 kg or <32 weeks), two included moderately preterm neonates (32 to 34 weeks) and two also included late preterm neonates (34 to 37 weeks). Four studies included a population of term neonates. Full details of all abstracted studies and reasons for exclusions are included in the Supplementary Table. Around one-third (nine) studies were from low- or middle-income countries (India, Malaysia, Mexico, Nepal, Turkey, Uruguay and Zambia). All studies were hospital based.
Effect on neonatal mortality
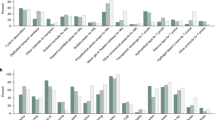
A meta-analysis of six RCTs examining very preterm neonates found no statistically significant difference in all-cause mortality (12.9 vs 17.0%; RR 0.73; 95% CI 0.48 to 1.13; Figure 2; Table 2).20, 25, 26, 27, 28, 29 Excluding three studies that used a radiant warmer instead of an incubator (in addition to the plastic wrap) during transport to a neonatal intensive care unit did not change the result (RR 0.86; 95% CI 0.49 to 1.49; Supplementary Table). Two RCTs including preterm infants of mixed gestation found no statistically significant reduction in neonatal mortality; one Malaysian study with neonates 24 to 34 weeks (10.0 vs 16.7%, RR 0.60; 95% CI 0.22 to 1.64)21 and one Zambian study with neonates aged 26 to 36 weeks (or <2.5 kg) (14.3 vs 5.5%, RR 2.62; 95% CI 0.72 to 9.58)23 (Table 2). Both studies were underpowered for this outcome. One RCT that examined mortality in term neonates reported no deaths in both the intervention and control groups.22
Six observational studies examining very preterm neonates also found no mortality benefit of being wrapped in plastic compared with blankets (RR 1.10; 95% CI 0.84 to 1.46)30, 31, 32, 33, 34, 35 (Table 2). The results were unchanged when one study, which did not use an incubator in transport, was excluded (RR 1.03; 95% CI 0.72 to 1.47).
Effect on neonatal morbidity
Three studies were found examining necrotizing enterocolitis in preterm infants. One RCT including preterm infants of mixed gestation found no strong evidence of a difference in rates of necrotizing enterocolitis between plastic covering and conventional care groups (4 vs 0%, RR 5.98; 95% CI 0.29 to 121.8).21 This was supported by the pooled estimate from two observational studies (24 vs 20%, RR 1.29; 95% CI 0.85 to 1.97).33, 34
Two RCTs reported on the association between plastic wraps and grades 3–4 intraventricular hemorrhage. Results did not reach statistical significance for mixed preterm infants (2 vs 7%, RR 0.30; 95% CI 0.03 to 2.60)21 or very preterm infants (15 vs 32%, RR 0.38; 95% CI 0.15 to 1.02).28 The pooled estimate from two observational studies also failed to reach statistical significance (19 vs 25%, RR 0.8; 95% CI 0.52 to 1.23).33, 34 Two RCTs on very preterm neonates found no evidence of a difference in other major brain injuries (10 vs 9%, RR 1.10; 95% CI 0.41 to 2.98).16, 27A third RCT on mixed preterm neonates also reported no major brain injuries.23
Plastic bags were not found to be associated with a reduced incidence of respiratory distress syndrome in an RCT of mixed preterm neonates (64 vs 62%, RR 1.04; 95% CI 0.78 to 1.38)21 or with a reduced incidence of bronchopulmonary dysplasia in an observational study of neonates <1000 g (28 vs 29%, RR 0.98; 95% CI 0.57 to 1.69).34
No study of term neonates reported on any morbidity outcome.
Effect on hypothermia
All identified RCTs defined hypothermia according to the World Health Organization’s definition of temperatures below 36.5 °C. Four RCTs in very preterm neonates found that the use of a plastic bag was associated with a 43% reduction in the risk of hypothermia (35 vs 61%, RR 0.57; 95% CI 0.46 to 0.71; Figure 3; Table 2).25, 26, 27, 28, 29 No appreciable change was noted on two sensitivity analyses that removed studies using an incubator or only assessed temperature rectally (Supplementary Table). Plastic wraps were also associated with a reduction in incidence of hypothermia in more mature neonates. One RCT of preterm neonates of 24 to 34 weeks gestation reported a 21% reduction (76 vs 97%, RR 0.79; 95% CI 0.67 to 0.93),21 and the pooled result of two RCTs in neonates from 26 to 36 weeks showed a 46% reduction (25 vs 49%, RR 0.54; 95% CI 0.36 to 0.79; Supplementary Table) in the incidence of hypothermia.23, 36 Ten identified observational studies reporting hypothermia incidence30, 31, 32, 33, 34, 37, 38, 39 did not use a standard definition for hypothermia, and thus were not pooled into a meta-analysis. No trend was observed based on gestational age or hypothermia cutoff used. Results ranged from a protective effect of 0 up to 85%.
Two studies assessed the effect of plastic bags on hypothermia in term neonates. Hypothermia was reduced by 24% (60 vs 73%, RR 0.76; 95% CI 0.6 to 0.96) in the RCT and by 58% (35 vs 85%, RR 0.42; 95% CI 0.32 to 0.55) in the observational study (Table 2).22, 40
Effect on temperature change
A total of 25 studies reported temperature change as an outcome. Seven RCTs found evidence that plastic wraps reduce heat loss by 0.66 °C in very preterm neonates (95% CI 0.43 to 0.90 °C).20, 21, 25, 26, 27, 28, 29 Conflicting results were noted across two RCTs including mixed preterm neonates; one reported no benefit (mean temperature difference 0.20 °C; 95% CI −0.26 to 0.66 °C),26 and a second reported a 1.26 °C difference (95% CI 1.06 to 1.46 °C).41 In addition, three RCTs including neonates from 26 to 37 weeks gestation had a pooled heat loss reduction of 0.4 °C (95% CI 0.25 to 0.55 °C; Supplementary Table).21, 23, 36 Three RCTs of term neonates reported temperatures between 0.2 °C and 0.8 °C higher with plastic wraps.22, 42, 43 These finding are supported by 12 observational studies.30, 31, 32, 33, 34, 35, 37, 38, 39, 44, 45, 46
Adverse effects including hyperthermia
As plastic coverings induce body temperature increase, there is a risk of hyperthermia associated with use. Eight RCTs reported on the number of preterm neonates who experienced hyperthermia after the plastic wrap intervention, with hyperthermia defined as temperatures above either 37.5 or 38.0 °C. Hyperthermia occurred in 1.5% (5/331) of control neonates (wrapped in cloth blankets) compared with 7.2% (six studies, 12/167) of very preterm neonates wrapped in plastic. There was no evidence of an increased risk with concurrent use of an incubator (5.4% with incubator use, 8.1% without, RR=0.66; 95% CI 0.19 to 2.3).20, 21, 25, 26, 27, 29 Hyperthermia was a rare outcome in all other gestational age groups, with no events (0/30) reported in an RCT of neonates <32 weeks,41 1.5% (2/132) incidence reported across four RCTs of neonates 26 to 37 weeks gestation21, 23, 26, 36 and 0.7% (1/135) incidence from one RCT of term neonates.22 In six observational studies of very preterm neonates, 8.6% (40/464) of those wrapped in plastic and 1.8% (14/759) of those wrapped in cloth blankets developed hyperthermia.30, 33, 34, 35, 37, 38
In all cases of hyperthermia, the neonates rapidly returned to normothermia when unwrapped from the plastic, with no ensuing morbidities or mortalities reported. No study reported any other adverse effects associated with the use of plastic wraps.
Discussion
Our results suggest that plastic bags reduce the risk of hypothermia by 21 to 46% among preterm neonates, with the greatest effect among the most preterm infants. This is consistent with previous estimates;10 however, it provides stronger support for this finding given that our meta-analyses included six RCTs. Our results also support previous findings of in-hospital prevalence of hypothermia (Table 2).5 In addition, we believe our review is the first to examine the use of plastic bags on term neonates. Two studies confirmed the high prevalence of hypothermia even in term neonates and reported a 24 to 58% reduction with the use of plastic wraps.
Our analyses did not find evidence for a reduction in mortality or morbidity with the use of plastic coverings.10, 14 However, lack of evidence does not equate to lack of effect, as these analyses were not powered for these outcomes. Our results also suggest that although the risk of hyperthermia is present, it is low (<5%). Furthermore, the risk did not seem to be appreciably greater with the concurrent use of an incubator. Caution must be taken in this result, given that it was also underpowered. Additional research is needed to see: (1) whether these important reductions in hypothermia translate on a population level to reduced morbidity and mortality; and (2) if plastic coverings are safe in low-resourced settings or at home, where continuous temperature monitoring and technological support for resuscitation are unavailable.
There were shared elements across the included studies that can help inform policy creation. All studies used plastic covering immediately after delivery without first drying the infant. The plastic stayed on for between 20 and 60 min or until normothermia was reached. In all cases, a radiant warmer was used in the delivery room, and then a radiant warmer or incubator was used to transfer the infant to the neonatal intensive care unit. Sensitivity analysis showed no difference in the rates of hypo- or hyperthermia between these two groups. There was no standardization of whether infants wore hats, the ambient room temperature or the type of plastic covering used. In addition, not all studies reported which parts of the body were covered with the plastic. One recent study comparing neonates who had their torsos covered in plastic versus torso and head found no difference in temperature, hypothermia or hyperthermia between the two groups.47 To guide policy development, it will be important to clarify whether there are differences in the efficacy or safety of plastic bags based on climate or the availability of other thermal care resources.
Five studies evaluated the efficacy of commonly available inexpensive materials (food packaging film or non-sterile grocery store bags), ideal for low-resource settings.22, 23, 38, 39, 45 The results from the studies cannot be pooled as they include subjects of varying gestational ages and had different study designs. However, each study reported a significant temperature difference, a reduction in hypothermia incidence and similar rates of hyperthermia to manufactured bags with no other adverse effects (Supplementary Table). More evidence must be collected to state conclusively whether these bags are as effective (with minimal danger posed by their non-sterility) as manufactured bags.
There are some limitations to this review. Most obviously, only one-third of included studies were from low- or middle-income countries, and all studies took place in hospitals with a neonatal intensive care unit. Therefore, these findings must be applied with caution to home births or primary care hospitals. Furthermore, no study compared the use of plastic bags with skin-to-skin care immediately following delivery. Skin-to-skin care is the current standard of care for stable neonates, as well as in transport between home and hospital.48 It has yet to be studied for use in immediate thermal stabilization. Both practices are low cost, accessible and could be used in low-resource settings. If plastic wraps were to be promoted in these settings, clear evidence of their benefits compared with skin-to-skin care would be required. Finally, in each study, plastic bags were used in different combinations with other thermal care practices. Therefore, we could not evaluate which combined intervention is most efficacious, or if plastic bags alone in the absence of any other heat device would be sufficient in preventing heat loss.
Despite decades of research on thermal care practices, neonatal hypothermia remains a common global problem, contributing to needless deaths.5 Our review provides strong support for the efficacy of plastic wraps to reduce heat loss and prevent hypothermia immediately following a hospital delivery in neonates of all gestational ages. Plastic wraps are available globally, can cost as little as US $0.03 per bag, are easy to use and carry a low risk of adverse events. Further research is required to assess their role outside hospital settings (for example, as part of birth kits), investigate morbidity or mortality decreases, as well as investigate instances other than delivery where they are useful.
References
Oza S, Cousens SN, Lawn JE . Estimation of daily risk of neonatal death, including the day of birth, in 186 countries in 2013: a vital-registration and modelling-based study. Lancet Glob Health 2014; 2 (11): e635–e644.
United Nations Inter-agency Group for Child Mortality Estimation. Levels and trends in child mortality. 2014. Available at http://www.datauniceforg/fckimages/uploads/1410869227_Child_Mortality_Report_2014pdf.
Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN . Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000-2013. Bull World Health Organ 2015; 93 (1): 19–28.
Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013; 382 (9890): 417–425.
Lunze K, Bloom DE, Jamison DT, Hamer DH . The global burden of neonatal hypothermia: systematic review of a major challenge for newborn survival. BMC Med 2013; 11: 24.
Mullany LC, Katz J, Khatry SK, LeClerq SC, Darmstadt GL, Tielsch JM . Risk of mortality associated with neonatal hypothermia in southern Nepal. Arch Pediatr Adolesc Med 2010; 164 (7): 650–656.
Bartels DB, Kreienbrock L, Dammann O, Wenzlaff P, Poets CF . Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed 2005; 90 (1): F53–F59.
Knobel RB, Vohra S, Lehmann CU . Heat loss prevention in the delivery room for preterm infants: a national survey of newborn intensive care units. J Perinatol 2005; 25 (8): 514–518.
Rosen HE, Oatley H, Blencowe H, Mullany LC, Kerber K, Baqui A et al. Thermal care practices for prevention of neonatal deaths: a systematic review and Delphi estimation of mortality effect. BMC Public Health 2013.
McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S . Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev 2010; (3): CD004210.
Gray PH, Flenady V . Cot-nursing versus incubator care for preterm infants. Cochrane Database Syst Rev 2011; (8): CD003062.
Conde-Agudelo A, Belizan JM, Diaz-Rossello J . Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011; (3): CD002771.
Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S . 'Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol 2010; 39 (Suppl 1): i144–i154.
Cramer K, Wiebe N, Hartling L, Crumley E, Vohra S . Heat loss prevention: a systematic review of occlusive skin wrap for premature neonates. J Perinatol 2005; 25 (12): 763–769.
Conde-Agudelo A, Belizan JM, Diaz-Rossello J . Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011; (3): CD002771.
Knobel RB, Vohra S, Lehmann CU . Heat loss prevention in the delivery room for preterm infants: a national survey of newborn intensive care units. J Perinatol 2005; 25 (8): 514–518.
Agourram B, Bach V, Tourneux P, Krim G, Delanaud S, Libert JP . Why wrapping premature neonates to prevent hypothermia can predispose to overheating. J Appl Physiol 2010; 108 (6): 1674–1681.
Moher D, Liberati A, Tetzlaff J, Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011, Available from www.cochrane-handbook.org.
Vohra S, Roberts RS, Zhang B, Janes M, Schmidt B . Heat loss prevention (HeLP) in the delivery room: a randomized controlled trial of polyethylene occlusive skin wrapping in very preterm infants. J Pediatr 2004; 145 (6): 750–753.
Rohana J, Khairina W, Boo NY, Shareena I . Reducing hypothermia in preterm infants with polyethylene wrap. Pediatr Int 2011; 53 (4): 468–474.
Belsches TC, Tilly AE, Miller TR, Kambeyanda RH, Leadford A, Manasyan A et al. Randomized trial of plastic bags to prevent term neonatal hypothermia in a resource-poor setting. Pediatrics 2013; 132 (3): e656–e661.
Leadford AE, Warren JB, Manasyan A, Chomba E, Salas AA, Schelonka R et al. Plastic bags for prevention of hypothermia in preterm and low birth weight infants. Pediatrics 2013; 132 (1): e128–e134.
StataCorp. (2013). Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP, 2013.
Knobel RB, Wimmer JE Jr., Holbert D . Heat loss prevention for preterm infants in the delivery room. J Perinatol 2005; 25 (5): 304–308.
Vohra S, Frent G, Campbell V, Abbott M, Whyte R . Effect of polyethylene occlusive skin wrapping on heat loss in very low birth weight infants at delivery: a randomized trial. J Pediatr 1999; 134 (5): 547–551.
Trevisanuto D, Doglioni N, Cavallin F, Parotto M, Micaglio M, Zanardo V . Heat loss prevention in very preterm infants in delivery rooms: a prospective, randomized, controlled trial of polyethylene caps. J Pediatr 2010; 156 (6): 914–917.
Moraes Castro M, Repeto M, Cancela MJ, Latof M, Hernández C, Bustos R . Experiencia clínica en la utilización de bolsa de polietileno para disminuir la hipotermia en el recién nacido menor de 1.000 gramos [Clinical experience in the use of polyethelene bags to reduce hypothermia in newborns less than 1000 g]. Arch Pediatr Urug 2007; 78 (2): 110–114.
Smith J, Usher K, Alcock G, Buettner P . Application of plastic wrap to improve temperatures in infants born less than 30 weeks gestation: a randomized controlled trial. Neonatal Netw 2013; 32 (4): 235–245.
Bredemeyer S, Reid S, Wallace M . Thermal management for premature births. J Adv Nurs 2005; 52 (5): 482–489.
Mathew B, Lakshminrusimha S, Cominsky K, Schroder E, Carrion V . Vinyl bags prevent hypothermia at birth in preterm infants. Indian J Pediatr 2007; 74 (3): 249–253.
Bjorklund LJ, Hellstrom-Westas L . Reducing heat loss at birth in very preterm infants. J Pediatr 2000; 137 (5): 739–740.
Carroll PD, Nankervis CA, Giannone PJ, Cordero L . Use of polyethylene bags in extremely low birth weight infant resuscitation for the prevention of hypothermia. J Reprod Med 2010; 55 (1-2): 9–13.
Billimoria Z, Chawla S, Bajaj M, Natarajan G . Improving admission temperature in extremely low birth weight infants: a hospital-based multi-intervention quality improvement project. J Perinat Med 2013; 41 (4): 455–460.
Singh A, Duckett J, Newton T, Watkinson M . Improving neonatal unit admission temperatures in preterm babies: exothermic mattresses, polythene bags or a traditional approach? J Perinatol 2010; 30 (1): 45–49.
Cardona Torres LM, Amador Licona N, Garcia Campos ML, Guizar-Mendoza JM . Polyethylene wrap for thermoregulation in the preterm infant: a randomized trial. Indian Pediatr 2012; 49 (2): 129–132.
Newton T, Watkinson M . Preventing hypothermia at birth in preterm babies: at a cost of overheating some? Arch Dis Child Fetal Neonatal Ed 2003; 88 (3): F256.
Ibrahim CP, Yoxall CW . Use of plastic bags to prevent hypothermia at birth in preterm infants—do they work at lower gestations? Acta Paediatr 2009; 98 (2): 256–260.
Lenclen R, Mazraani M, Jugie M, Couderc S, Hoenn E, Carbajal R et al. Utilisation d’un sac en polyéthylène: un moyen d’améliorer l’environnement thermique du prématuré en salle de naissance. [Use of a polyethylene bag: a way to improve the thermal environment of the premature newborn at the delivery room]. Arch Pediatr 2002; 9 (3): 238–244.
Johanson RB, Spencer SA, Rolfe P, Jones P, Malla DS . Effect of post-delivery care on neonatal body temperature. Acta Paediatr 1992; 81 (11): 859–863.
Gathwala G, Singh G, Agrawal N . Safety and efficacy of vinyl bags in prevention of hypothermia of preterm neonates at birth. Indian J Public Health 2010; 54 (1): 24–26.
Besch NJ, Perlstein PH, Edwards NK, Keenan WJ, Sutherland JM . The transparent baby bag. A shield against heat loss. N Engl J Med 1971; 284 (3): 121–124.
Raman S, Shahla A . Temperature drop in normal term newborn infants born at the University Hospital, Kuala Lumpur. Aust N Z J Obstet Gynaecol 1992; 32 (2): 117–119.
Meyer MP . Swaddling and heat loss. Arch Dis Child Fetal Neonatal Ed 2003; 88 (3): F256.
Duman N, Utkutan S, Kumral A, Koroglu TF, Ozkan H . Polyethylene skin wrapping accelerates recovery from hypothermia in very low-birthweight infants. Pediatr Int 2006; 48 (1): 29–32.
Castrodale V, Rinehart S . The golden hour: improving the stabilization of the very low birth-weight infant. Adv Neonatal Care 2014; 14 (1): 9–14.
Doglioni N, Cavallin F, Mardegan V, Palatron S, Filippone M, Vecchiato L et al. Total body polyethylene wraps for preventing hypothermia in preterm infants: a randomized trial. J Pediatr 2014; 165 (2): 261–266.e1.
Chantaroj S, Techasatid W . Effect of polyethylene bag to prevent heat loss in preterm infants at birth: a randomized controlled trial. J Med Assoc Thai 2011; 94 (Suppl 7): S32–S37.
Acknowledgements
Funding was provided by the World Health Organization as part of systematic reviews for guidelines for preterm care, and Children’s Investment Fund Foundation as part of analysis for Every Newborn Action Plan. This review was funded by the Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Oatley, H., Blencowe, H. & Lawn, J. The effect of coverings, including plastic bags and wraps, on mortality and morbidity in preterm and full-term neonates. J Perinatol 36 (Suppl 1), S83–S89 (2016). https://doi.org/10.1038/jp.2016.35
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.35
This article is cited by
-
Embrace versus Cloth Wrap in preventing neonatal hypothermia during transport: a randomized trial
Journal of Perinatology (2021)
-
Assessment of rewarming methods in unplanned out-of-hospital births from a prospective cohort
Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine (2020)
-
Significant factors influencing inadvertent hypothermia in pediatric anesthesia
Journal of Clinical Monitoring and Computing (2019)
-
Golden hour of neonatal life: Need of the hour
Maternal Health, Neonatology and Perinatology (2017)
-
Intraoperative temperature regulation in children using a liquid-warming garment
Pediatric Surgery International (2017)