Abstract
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of chlorhexidine application to the umbilical cord in neonates. We searched MEDLINE and other electronic databases, and included all RCTs that evaluated the effect of single or multiple chlorhexidine cord applications on the neonatal mortality rate (NMR) and/or the incidence of systemic sepsis and omphalitis. A total of six RCTs—four community-based cluster RCTs and two hospital-based trials—were included in the review. Of the four cluster RCTs, three were conducted in South Asia in settings with high rates of home births (>92%) while the fourth, available only as an abstract, was conducted in Africa. Pooled analysis by the ‘intention-to-treat’ principle showed a significant reduction in NMR after chlorhexidine application (four studies; relative risk (RR) 0.85; 95% confidence interval (CI) 0.76 to 0.95; fixed effects (FE) model). On subgroup analysis, only multiple applications showed a significant effect (four studies; RR 0.88; 95% CI 0.78 to 0.99) whereas a single application did not (one study; RR 0.86; 0.73 to 1.02). Similarly, only the community-based trials showed a significant reduction in NMR (three studies; RR 0.86; 95% CI 0.77 to 0.95), while the hospital-based study did not find any effect (RR 0.11; 0.01 to 2.03). Since all the studies were conducted in high-NMR settings (⩾30 per 1000 live births), we could not determine the effect in settings with low NMRs. Only one study—a hospital-based trial from India—reported the incidence of neonatal sepsis; it did not find a significant reduction in any sepsis (RR 0.67; 95% CI 0.35 to 1.28). Pooled analysis of community-based studies revealed significant reduction in the risk of omphalitis in infants who received the intervention (four studies; RR 0.71; 95% CI 0.62 to 0.81). The hospital-based trial had no instances of omphalitis in either of the two groups. Chlorhexidine application delayed the time to cord separation (four studies; mean difference 2.11 days; 95% CI 2.07 to 2.15; FE model). Chlorhexidine application to the cord reduces the risk of neonatal mortality and omphalitis in infants born at home in high-NMR settings. Routine chlorhexidine application, preferably daily for 7 to 10 days after birth, should therefore be recommended in these infants. Given the paucity of evidence, there is presently no justification for recommending this intervention in infants born in health facilities and/or low-NMR settings.
Similar content being viewed by others
Introduction
Deaths in the neonatal period, that is, the first 28 days of life, account for about 40% of all deaths in children younger than 5 years.1 Achieving Millennium Development Goal 4, which stipulates a reduction of two thirds in deaths in children under 5 years of age by 2015,2 would therefore require a substantial reduction in the neonatal mortality rate (NMR).
Of the 3.1 million neonatal deaths that occur globally every year, infections account for about 30%.1 In settings with high-mortality rates, the proportion due to infections is even greater.3 Such high-infection-related death rates make it imperative to identify simple and cost-effective interventions that can be implemented in different settings across the globe.4 Use of topical antiseptics is one intervention that could reduce the incidence of infections by preventing or reducing the bacterial colonization of the skin or umbilical cord in neonates.
Of the different antiseptics available, chlorhexidine is the most studied agent in newborn infants. Research has shown a significant reduction in the rates of bacterial colonization of the umbilical cord after chlorhexidine application(s).5, 6 However, the effect of chlorhexidine application on the rates of omphalitis or systemic sepsis is less clear. A Cochrane review on topical antiseptics for umbilical cord care, which included one study on chlorhexidine application, did not find any benefit.7 In the absence of evidence for the efficacy of chlorhexidine or other topical antiseptics, the World Health Organization currently advocates dry cord care for infants born in developing countries.8
New evidence from randomized controlled trials (RCTs) conducted in Bangladesh, Nepal and Pakistan has been published in the last few years.9, 10, 11 A recent systematic review including these studies concluded that cord chlorhexidine application reduced neonatal mortality by 17% (pooled relative risk (RR): 0.83; 95% confidence interval (CI) 0.74 to 0.94).12 However, the review had major limitations13—first, the included cluster RCTs analyzed the data of only those infants who were seen by the investigators in the first 7 to 10 days of life and therefore did not report the results of intention-to-treat (ITT) analysis. Second, the reviewers failed to adjust for ‘design effect’, an important consideration given that all three studies were cluster RCTs. A recent Cochrane review also reported that cord chlorhexidine reduces neonatal mortality by 23% (RR 0.73; 95% CI 0.63 to 0.94) and omphalitis by 27–56%, depending upon the severity.14 The review also failed to address the issue of post-randomization exclusion of some neonates in the three community-based cluster trials. More recently, a hospital-based study from India was published.15, 16
Considering these limitations as well as the potential public health importance of emerging evidence on umbilical cord care, we conducted a systematic review to evaluate the effect of topical application of chlorhexidine to the umbilical cord on NMR and the incidence of systemic sepsis and omphalitis in the neonatal period to provide updated evidence for policy makers and other stakeholders.
Methods
Types of studies
We included all RCTs, including cluster randomized trials and quasi-RCTs, which compared topical application of chlorhexidine with the umbilical cord with dry cord or ‘usual’ cord care practices in this review.
Types of participants
All studies that enrolled infants in the first 28 days of life at either health facilities or in the community were eligible for inclusion in the review.
Types of interventions
Studies that compared the effect of single or multiple applications of chlorhexidine to the umbilical cord with dry cord care or the ‘usual’ cord care practices were included in the review. We excluded studies that compared application of chlorhexidine with that of other topical antiseptics as well as the studies that used chlorhexidine in combination with another antiseptic such as alcohol or iodine.
Outcome measures and their definitions
Our primary outcome variable was the NMR, defined as the number of deaths in the first 28 days of life per 1000 live births. Our secondary outcome variables were the incidences of systemic sepsis, omphalitis and adverse neurodevelopmental outcomes, and the time to separation of the umbilical cord. Systemic sepsis was defined as the presence of clinical features of sepsis/meningitis with or without isolation of organisms from an otherwise sterile site and laboratory parameters suggestive of sepsis (such as decreased total white cell or absolute neutrophil count, positive C-reactive protein and so on). Omphalitis was defined as the presence of redness or swelling, with or without pus, in the skin surrounding the umbilical cord stump. Adverse neurodevelopmental outcome was defined as the presence of cerebral palsy or moderate to severe developmental delay as assessed by performance in formal neurodevelopmental testing at 18 to 24 months of age.
Search methods for identification of studies
We initially searched the electronic bibliographic databases including MEDLINE, Embase, Cochrane CENTRAL, Web of Science, CINAHL, WHOLIS and the clinical trials website (www.clinicaltrials.gov) up to April 2012. We used the following terms for searching MEDLINE: (‘chlorhexidine’ (MeSH Terms) OR ‘chlorhexidine’ (All Fields)) AND ((‘infant, newborn’ (MeSH Terms) OR (‘infant’ (All Fields) AND ‘newborn’ (All Fields)) OR ‘newborn infant’ (All Fields) OR ‘neonates’ (All Fields)). Similar terms were used for searching the other databases. No language restrictions were applied. We later updated the search to December 2014. However, due to practical reasons we could only carry out the update in MEDLINE, Cochrane CENTRAL and the clinical trials website.
We scanned the title and abstract of the retrieved citations to exclude those that were obviously irrelevant. We retrieved the full text of the remaining studies to identify relevant articles.
Data extraction and management
Data extraction was carried out using a form designed and pilot tested by the authors. Three authors (MJS, AC and AR) independently extracted the study setting, study intervention, practices in the control group, sample size, randomization procedure, risk of bias and the outcomes of interest. For categorical outcomes, we extracted the total number of participants for each group, the number of participants experiencing an event, and the adjusted RR and its 95% CI. In factorial trials and in multi-arm studies with two or more intervention groups and a single control group, we combined all relevant experimental intervention groups of the study into a single group and all relevant control intervention groups into a single control group to create a single pair-wise comparison. This approach, recommended by the Cochrane handbook, prevents unit-of-analysis error.17 For the continuous outcome, mean, s.d. and total number of participants in each group were extracted.
Quality assessment of the randomized trials was undertaken using the standard criteria of allocation concealment, blinding and completeness of follow-up (classified as yes, no or unclear). Disagreements between the authors were resolved by consensus as well as discussion with the senior author (VKP).
Subgroup and sensitivity analysis
We examined the effect of chlorhexidine application to the cord on the primary outcome in the following sub-groups:
-
1
RCTs vs quasi-randomized studies
-
2
Home vs facility births
-
3
High-NMR (⩾30 per 1000 live births) vs lower NMR (<30 per 1000 live births) setting
-
4
Infants receiving unhygienic cord care practices vs those who received dry cord care
-
5
Single vs multiple applications
-
6
Low birth weight vs normal birth weight.
Statistical analysis
Data entry and meta-analysis were performed with user-written programs on Stata 11.2 software (Stata Corp, College Station, TX, USA). Pooled estimates of the outcome measures were calculated from the RRs and 95% CIs/standard error of the individual studies by the generic inverse variance method with the user-written ‘metan’ command in Stata.18 In a hierarchical pattern, we gave preference to the adjusted RR obtained by the study authors in the ITT analysis. When trials did not provide the result of ITT analysis, we reconstructed it using the relevant data from the trial flow of the individual studies. For cluster RCTs, the RR and 95% CI obtained by this method were adjusted for the cluster design using a design effect inflation of standard error.19 We used the design effect provided in the studies for this adjustment. For one study that did not provide the design effect,11 we estimated it by permutation, that is, by using different effect sizes and then selecting the one which, when used to inflate the standard error, provided the same RR and 95% CI as that mentioned in the study results. If the relevant data were not available to reconstruct the ITT, we used the adjusted RRs or mean differences provided in the study to calculate the pooled result.
We computed the pooled estimates by using both fixed effects (FE) and random effects (RE) model assumptions and expressed them in an exponential form. The heterogeneity between the studies was quantified by using a measure of the degree of inconsistency in their results (I2 statistic). We carried out sensitivity and subgroup analyses for the primary outcome—NMR—by disaggregating results with the user-written ‘metan’ command (‘by option’) in Stata software.18 We also evaluated the presence of publication bias in the extracted data for the primary outcome using funnel plots.19
Results
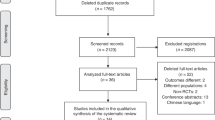
We retrieved 666 citations, of which 630 were excluded after screening the title and abstract. Of the remaining 36 studies, six were found to be eligible for inclusion in the review (Figure 1).9, 10, 11, 16, 20, 21
Table 1 summarizes the characteristics of the included studies. Four were cluster RCTs conducted in community settings in South Asia (n=3)9, 10, 11 or Africa (n=1).21 The studies from South Asia included mostly home births; the predominant place of delivery is not known for the study from Africa (available only in abstract form).22 The remaining two studies were conducted in hospital settings, one each in a developed (Germany)20 and developing country (India).15, 16, 20 The four community trials used 4% chlorhexidine solution whereas the hospital-based trials evaluated the efficacy of chlorhexidine dry powder20 or 2.5% chlorhexidine solution.16 One cluster RCT compared the effect of single as well as multiple applications of chlorhexidine with dry cord care;10 the remaining studies evaluated only multiple applications of chlorhexidine (Table 1).9, 11, 16, 20, 21
Table 2 shows the risk of bias in the included studies. The outcome assessors were not blinded to the intervention in all but one of the studies. The risk of measurement bias appears low given the objective nature of the primary outcome (NMR); the same is not true for other outcomes. None of the three cluster RCTs, for which complete information was available, reported the results of ITT analysis. The attrition bias was also high (>10%) in two of these studies.10, 11 One of the hospital-based studies had methodological limitations in both follow-up and analysis (Table 2).20
Primary outcome; NMR
Of the six studies, only four—the three cluster RCTs and one hospital-based trial from South Asia—provided data on NMR. All four studies were conducted in high-NMR settings (the range in the control groups was 34 per 1000 live births to 57 per 1000 live births). Two studies also reported a high prevalence of unhygienic cord care practices, ranging from 54 to 90% (Table 1). The three community trials enrolled predominantly home births (92 to 100%). All three studies provided data for only those infants who received the intervention—either partially or fully—and not for all infants who were in the randomized clusters. For the purpose of this review, we used the data of all infants in the randomized clusters, irrespective of whether they received the intervention, so as to reconstruct the ITT analysis.23 Table 3 depicts the data from the individual studies that were used in the present review.9, 10, 11
We observed substantial heterogeneity upon pooling the results of the three studies (I2=67.9%; Figure 2). Pooled analysis by FE model showed a significant reduction in NMR in the intervention group (RR 0.85; 95% CI 0.76 to 0.95) whereas that by the RE model did not show any significant benefit (RR 0.78; 95% CI 0.61 to 1.0; Figure 2). The number needed to treat based on the average control risk (3.5%) was 200 (95% CI 125 to 500). As the number of studies was small, we did not produce a funnel plot to examine the presence of publication bias.
Subgroup analysis
Single vs multiple applications
Similar to the overall results, pooled analysis of the three studies that compared multiple chlorhexidine applications with dry cord care showed a significant difference in NMR by the FE model (RR 0.88; 95% CI 0.78 to 0.99) but not in the RE model (RR 0.79; 95% CI 0.59 to 1.06). There was substantial heterogeneity between the results of the individual studies (I2=74.2%). The only study that evaluated a single chlorhexidine application did not show any significant reduction in the mortality rate of all randomized infants (RR 0.86; 95% CI 0.73 to 1.02).10
Low birth weight vs normal birth weight infants
None of the included studies provided information on the NMRs of low birth weight compared with normal birth weight infants. One study provided the birth weight-specific mortality rates of infants who were seen by the study team in the first 7 to 10 days.10 There was no difference in mortality rates between the single/multiple chlorhexidine application(s) and the dry cord care groups in either low birth weight (RR 0.94; 95% CI 0.75 to 1.18) or normal birth weight infants (RR 0.90; 95% CI 0.62 to 1.32).
Facility versus home births
A hospital-based study from India did not find any effect on NMR following cord chlorhexidine application (RR 0.11; 95% CI 0.01 to 2.03). No information was available on the mortality rates of infants born at home compared with those born in health facilities in the community-based trials. However, the proportion of facility births in these studies was very small (0 to 8%).9, 10, 11
Low-NMR (<30 per 1000 live births) vs high-NMR (⩾30 per 1000 live births) settings
We could not examine the effect sizes in the low-NMR and high-NMR settings separately as all the studies were conducted in high-NMR settings (the NMR of the control group being ⩾30 per 1000 live births).
Unhygienic cord care practices vs dry cord care
We did not have enough information on the RRs of neonatal mortality in the sub-groups of infants receiving unhygienic cord care practices or dry cord care.
Secondary outcomes
Incidence of systemic sepsis
Only the hospital-based study from India reported this outcome. There was no significant reduction in the incidence of any sepsis, that is, culture positive or culture negative (RR 0.67; 95% CI 0.35 to 1.28). Interestingly, the authors reported a significantly lower incidence of culture-positive sepsis in the chlorhexidine group (2.8% vs 21.4%; RR 0.13; 95% CI 0.03 to 0.56).15
Omphalitis
Five studies reported the incidence of omphalitis.9, 10, 11, 15, 20 None of the three cluster RCTs stated the results of ITT analysis. As we did not have the complete data to reconstruct the ITT analysis for omphalitis, we used the RRs provided in the study results (non-ITT analysis). Pooled analysis of four studies revealed a significant reduction in the incidence of omphalitis by both FE and RE models in infants who received the intervention (RR 0.71; CI 0.62 to 0.81 and 0.70; 0.61 to 0.82, respectively; Figure 3). The fifth study from India did not find any cases of omphalitis in either of the two groups.15
Time to cord separation
All six studies reported the time to cord separation in enrolled infants. Pooled analysis of four studies showed a prolongation in time to cord separation by 2.11 days (95% CI 2.07 to 2.15) and 1.44 days (95% CI 0.51 to 2.37) using FE and RE models, respectively (Figure 4). There was considerable heterogeneity in this outcome (I2=99.6%) with one study reporting shorter time to separation of cord in the chlorhexidine arm,16 while all the others reported delayed separation.10, 22, 24 Information from two studies could not be used in the pooled analysis because of incomplete data. The hospital-based trial from Germany found a significant delay in separation of the cord in the intervention group, the mean difference being 18.9 h (95% CI not provided).20 In contrast, the factorial RCT from Pakistan reported no difference in time to separation of the cord between the groups (mean time of separation ranged from 5.9 to 6.2 days in all four groups).11
Adverse neurodevelopmental outcomes
None of the studies reported this outcome.
Discussion
Given the limitations of the two recently published systematic reviews12, 14 (see Introduction section), the findings of the present review assume great importance for both health professionals and policy makers. Pooled analysis by the FE model revealed significant reductions in the NMR following chlorhexidine application to the cord. However, the pooled estimate by RE model did not yield a significant result. The discrepancy between the two estimates was due to the substantial heterogeneity observed between the included studies (I2=69.2%). We did not observe any evidence of major clinical heterogeneity such as differences in the study setting, population, intervention or in the outcome variable between the three community-based studies that contributed to >99% of weight in the pooled analysis. The statistical heterogeneity that led to the discrepancy is also due to the difference in the magnitude rather than in the direction of the effect sizes of the individual studies (Figure 2). Given these considerations, as well as the fact that the RE estimate does not reflect the actual effect in any particular population being studied,25 pooled RR by the FE model may be considered as the best estimate of the ‘real’ effect. Accordingly, cord chlorhexidine application might be expected to reduce the NMR by about 15% (RR 0.85; 95% CI 0.76 to 0.95) in infants born in settings that are comparable to the study settings. The intervention was also found to reduce the incidence of omphalitis by almost 30% (Figure 3).
Do these beneficial effects following chlorhexidine application warrant a change in the current recommendation on cord care in neonates? The question was answered by Osrin and Hill26 in their editorial on chlorhexidine cord cleansing—‘….if the need is clear, the possibilities attractive, and the risk low, how much evidence is necessary before we act on plausible findings?’. However, this would mean a paradigm shift from the existing policy of dry cord care to routine application of chlorhexidine in infants born in any setting across the globe. Also, it would have huge implications in terms of costs and manpower required for delivery of the intervention, particularly in developing country settings.
The currently available evidence has three major pitfalls. First, the generalizability of the results is low. The three community trials from South Asia, which contributed to almost the entire weight in the pooled analysis, were conducted in settings that had high NMR (⩾30 per 1000 live births in the control group) and uniformly low rates of institutional births. Two of the studies reported a high prevalence of unhygienic cord care practices such as application of oil, ash and so on.9, 11 Interestingly, these studies showed a significant effect on both NMR and omphalitis,9, 11 while the third study that reported a low prevalence (<7%) of these practices did not show any benefit in either of these outcomes (Figures 2 and 3).10 The fourth study, a hospital-based trial from India with possibly low rates of unhygienic cord care practices, also had a high baseline NMR in the control arm (57.1 per 1000 live births).16 This study did not find any significant benefit in the risk of mortality or omphalitis but showed a reduction in the incidence of culture-positive sepsis. Clearly, the results from these high-risk settings cannot be generalized to settings with low NMR or with high rates of institutional births.
Second, the magnitude of reduction in NMR after the intervention was only modest, at about 15% (Figure 2). This is likely to be an overestimate of the ‘real’ effect as we could adjust the effect sizes of the individual studies for only the cluster design and not for other potential confounders. In contrast, the reported results of non-ITT analysis of the cluster RCTs showed much higher benefit, the estimated reduction ranging from 20% to 38% (except for the multiple applications group in one study that showed no significant effect).10 Not surprisingly, the Cochrane review that included these trials also found a 23% reduction in NMR with chlorhexidine application.14 This discrepancy is due to the different methods of analysis used in the current review compared with the individual studies. Although we used the data of all infants in the randomized clusters, the study investigators analyzed the data of only those infants who were seen by the study team in the first 7 to 10 days9, 10 and/or born at home and consented to participate in the study.11 The latter approach, also adopted by the authors of two systematic reviews,12, 14 has major flaws. First, it is not a true ITT analysis as many infants were excluded after randomization. In a cluster RCT, all the infants born in the randomized clusters are considered study subjects, irrespective of whether they received the intervention or not. The outcomes of all these infants should be taken into account in the ITT analysis.27, 28 Inclusion of all enrolled infants would reduce the risk of selection bias. In contrast with the approach adopted by the study investigators, one cannot reliably exclude the risk of differential recruitment between the groups. Second, analysis methods used by the study authors spuriously inflate the magnitude of reduction in neonatal mortality by excluding the deaths that occurred before the visit of the study team. Although deaths in the first few days of life are most often due to perinatal asphyxia and prematurity, the deaths that occur later are caused predominantly by sepsis. Excluding early deaths would therefore magnify the effect of the intervention on the remaining deaths in the later neonatal period. The magnitude of reduction in neonatal mortality reported in the individual studies (20 to 38%)9, 10, 11 actually refers to the deaths that happened after the first 1 or 2 days of life and not the overall NMR. About one half of all neonatal deaths occur in the first 2 days of life.29
Third, there is little evidence on the safety of chlorhexidine application in neonates, particularly in preterm infants. None of the studies evaluated the effect of the intervention on long-term neurodevelopmental outcomes. There is a paucity of data on the absorption of chlorhexidine from the cord or skin in neonates. The reviews that measured serum levels following whole-body chlorhexidine application concluded that some percutaneous absorption occurs in preterm neonates.30, 31 Even though the risk of absorption is low with cord application(s), it cannot be completely ruled out.
Implications for policy makers
The aforementioned pitfalls indicate that the risk-benefit ratio of the intervention is likely to vary for infants born in different settings. Given that chlorhexidine application reduces the NMR by about 15%, policy makers and other stakeholders from high-NMR settings are likely to give a high value to the intervention even in the absence of data on safety outcomes. On the other hand, those from low-NMR settings (<30 per 1000 live births) and/or settings with high rates of institutional births are likely to be skeptical given the lack of evidence for efficacy and safety of the intervention in their circumstances.
Implications for researchers
There is an urgent need to evaluate the effect of chlorhexidine application in low-NMR settings, and in institutional births in high-NMR settings. Two ongoing trials in Africa may provide the necessary evidence for this region.14, 29 They should also help the understanding of regional differences, if any, in the efficacy of the intervention. Given that the intervention could potentially reduce the incidence of culture-positive sepsis in hospital-born infants, there is a need for large multi-center trials that evaluate the effect of cord chlorhexidine application in facility births. Future research should also examine the optimal frequency of chlorhexidine application in high-NMR settings. In the present review, multiple chlorhexidine applications were found to reduce the NMR, while a single application did not show any significant benefit. However, one trial with a small sample size examined the effect of a single application whereas three studies evaluated the efficacy of multiple applications. There is also a need to carefully document the safety outcomes of chlorhexidine cord application when it is implemented on a larger scale.
Strengths and weaknesses
We have attempted to synthesize and update the evidence on chlorhexidine cleansing of the umbilical cord, one of the few interventions that has shown an impact on neonatal mortality in recent times. We used a rigorous methodology that minimizes the risk of bias in the analysis to provide the ‘true’ estimate of the effect of the intervention. As we did not have information on outcomes of infants who were lost to follow-up, the method of analysis might not qualify as ITT. The alternative approaches in such a scenario are to impute values making assumptions about the final outcomes or to use the ‘available case analysis'. Considering the problems associated with the former method, the Cochrane handbook recommends the latter approach.17
The present review has many limitations. First, we did not use the RRs adjusted for all potential confounders in the pooled analysis, as we could not obtain this information from the study investigators. Similarly, we had to use the RRs of omphalitis obtained by non-ITT analysis rather than that by ITT. Consequently, the pooled effect sizes of both NMR and omphalitis are likely to be an overestimate of the real effect. Second, we were unable to ascertain the effect of the intervention in pre-specified sub-groups such as unhygienic cord care practices compared with dry cord care as well as high-NMR compared with low-NMR settings due to lack of adequate information. This analysis could have helped identify the group of neonates who are more likely to benefit from the intervention. Third, we combined the data of the two intervention groups—multiple and single applications of chlorhexidine—in the study from Bangladesh10 and then used it in the pooled analysis for the secondary outcome, time to separation of cord. This method could have resulted in spuriously narrow CIs and consequently more weight for this study. However, this was inevitable given the nature of the interventions used in the study.
Conclusions
Chlorhexidine application to the cord reduces the risk of neonatal mortality and omphalitis in infants born at home in high-NMR settings. Therefore, routine chlorhexidine application, preferably daily for 7 to 10 days after birth, is recommended in these infants. There is presently insufficient evidence for recommending this intervention in infants born in health facilities and/or low-NMR settings.
References
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379 (9832): 2151–2161.
Haines A, Cassels A . Can the millennium development goals be attained? Br Med J 2004; 329 (7462): 394–397.
Lawn JE, Cousens S, Bhutta ZA, Darmstadt GL, Martines J, Paul V et al. Why are 4 million newborn babies dying each year? Lancet 2004; 364 (9432): 399–401.
Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA . Hospital-acquired neonatal infections in developing countries. Lancet 2005; 365 (9465): 1175–1188.
Meberg A, Schoyen R . Bacterial colonization and neonatal infections. Effects of skin and umbilical disinfection in the nursery. Acta Paediatr Scand 1985; 74 (3): 366–371.
Seeberg S, Brinkhoff B . Epidemiology and control of staphylococcal pyoderma among newborn-infants—evaluation of a method for routine cord care with 4-percent chlorhexidine-detergent solution. J Hosp Infect 1984; 5 (2): 121–136.
Zupan J, Garner P, Omari Aika AA . Topical umbilical cord care at birth. Cochrane Database Syst Rev 2004; 3: CD001057.
Iannotti LL, Zavaleta N, Leon Z, Caulfield LE . Growth and body composition of Peruvian infants in a periurban setting. Food Nutr Bull 2009; 30 (3): 245–253.
Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S et al. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 2006; 367 (9514): 910–918.
Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet 2012; 379 (9820): 1022–1028.
Soofi S, Cousens S, Imdad A, Bhutto N, Ali N, Bhutta ZA . Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. Lancet 2012; 379 (9820): 1029–1036.
Karumbi J, Mulaku M, Aluvaala J, English M, Opiyo N . Topical umbilical cord care for prevention of infection and neonatal mortality. Pediatr Infect Dis J 2013; 32 (1): 78–83.
Chandrasekaran A, Sankar MJ, Agarwal R, Paul VK . Topical umbilical cord care. Pediatr Infect Dis J 2013; 32 (7): 801.
Imdad A, Bautista RM, Senen KA, Uy ME, Mantaring JB 3rd, Bhutta ZA . Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database Syst Rev 2013; 5: CD008635.
Gathwala G, Sharma D, Bhakhri B . Effect of topical application of chlorhexidine for umbilical cord care in comparison with conventional dry cord care on the risk of neonatal sepsis: a randomized controlled trial. J Trop Pediatr 2013; 59 (3): 209–213.
Sharma D, Gathwala G . Impact of chlorhexidine cleansing of the umbilical cord on cord separation time and neonatal mortality in comparison to dry cord care—a nursery-based randomized controlled trial. J Matern Fetal Neonatal Med 2014; 27 (12): 1262–1265.
Higgins JPT, Deeks JJ, Altman DG . Special topics in statistics. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of interventions Version 5.1.0. The Cochrane Collaboration: March 2011. Available from www.cochrane-handbook.org, 2011.
Stern JAC, Bradburn MJ, Egger M . Meta-analysis in STATA TM. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Publishing: London, 2001, pp 347–369.
Sterne JAC, Bradburn MJ, Egger M . Meta-analysis in STATA TM. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Publishing: London, UK, 2001, pp 189–208.
Kapellen TM, Gebauer CM, Brosteanu O, Labitzke B, Vogtmann C, Kiess W . Higher rate of cord-related adverse events in neonates with dry umbilical cord care compared to chlorhexidine powder. Neonatology 2009; 96 (1): 13–18.
Zambia Chlorhexidine Application Trial (ZamCAT). ClinicalTrials.gov Identifier: NCT01241318. Available at http://clinicaltrials.gov/ct2/show/study/NCT01241318 (accessed on 31 December 2013).
Herlihy JM, Semrau K, Mazimba A et al. Chlorhexidine 4% umbilical wash lengthens time to cord separation. Pediatric Academic Societies Annual Meeting, Boston, 2012.
Donner A, Klar N (eds). Design and Analysis of Cluster Randomization Trials in Health Research. Arnold Publishers: London, 2000.
Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Katz J, Tielsch JM . Impact of umbilical cord cleansing with 4.0% chlorhexidine on time to cord separation among newborns in Southern Nepal: a cluster-randomized, community-based trial. Pediatrics 2006; 118 (5): 1864–1871.
Deeks JJ, Higgins JPT, Altman DG . Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration: March 2011. Available from www.cochrane-handbook.org.
Osrin D, Hill ZE . Chlorhexidine cord cleansing to reduce neonatal mortality. Lancet 2012; 379 (9820): 984–986.
Campbell MJ . Cluster randomized trials in general (family) practice research. Stat Methods Med Res 2000; 9 (2): 81–94.
Puffer S, Torgerson D, Watson J . Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. Br Med J 2003; 327 (7418): 785–789.
ICMR Young Infant Study Group. Age profile of neonatal deaths. Indian Pediatr 2008; 45 (12): 991–994.
Mullany LC, Darmstadt GL, Tielsch JM . Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J 2006; 25 (8): 665–675.
Chapman AK, Aucott SW, Milstone AM . Safety of chlorhexidine gluconate used for skin antisepsis in the preterm infant. J Perinatol 2012; 32 (1): 4–9.
Acknowledgements
We thank Dr Rajiv Bahl, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, Geneva, Switzerland, for his highly valuable comments on the methodology of the review. This work was funded by the Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
Author contributions
MJS modified the protocol, extracted data, did the statistical analysis and wrote the manuscript. AC prepared the study protocol, applied the search strategy, retrieved the articles, extracted data and helped in writing the manuscript. AR applied the search strategy, extracted data and helped in writing the manuscript. RA modified the study protocol, supervised data extraction, helped in statistical analysis and finalized the manuscript. VKP guided development of the protocol, supervised data extraction and finalized the manuscript. MJS, RA and VKP act as the guarantors of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Sankar, M., Chandrasekaran, A., Ravindranath, A. et al. Umbilical cord cleansing with chlorhexidine in neonates: a systematic review. J Perinatol 36 (Suppl 1), S12–S20 (2016). https://doi.org/10.1038/jp.2016.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.28
This article is cited by
-
RETRACTED ARTICLE: Application of 4% chlorhexidine to the umbilical cord stump of newborn infants in lower income countries: a systematic review and meta-analysis
Maternal Health, Neonatology and Perinatology (2019)
-
Causes of death in preterm neonates (<33 weeks) born in tertiary care hospitals in India: analysis of three large prospective multicentric cohorts
Journal of Perinatology (2019)
-
What is the result of vaginal cleansing with chlorhexidine during labour on maternal and neonatal infections? A systematic review of randomised trials with meta-analysis
BMC Pregnancy and Childbirth (2018)
-
Skin care practices in newborn nurseries and mother–baby units in Maryland
Journal of Perinatology (2017)