Abstract
Objective:
Lipid peroxides (LPOs) are released when free radicals react with unsaturated fatty acids in cell membranes during hypoxic ischemic insult in neonates. We aimed to assess LPO concentrations in the serum of asphyxiated and non-asphyxiated neonates and examine their correlation with the severity of asphyxia.
Study Design:
This prospective cross-sectional study was conducted on a group of asphyxiated neonates and controls. Serum LPO concentrations was measured by enzyme-linked immunosorbent assay at 4–6 h of life in all subjects. Encephalopathy was classified according to Sarnat’s stages into mild, moderate and severe at 12–24 h of life. LPO was compared between groups and was correlated with severity of encephalopathy and mortality.
Results:
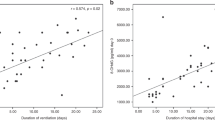
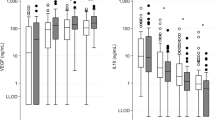
A total of 90 infants were enrolled; of them 45 had asphyxia. Serum LPO (nmol ml−1) was significantly greater in the asphyxia group (6.9±3.01 vs 1.78±1.09, P<0.001). It correlated positively with severity of encephalopathy (P<0.001) and negatively with Apgar score at 5 min (r=−0.532, P<0.001) and with initial blood gases pH (r=−0.664, P<0.001). LPO measured greater concentrations in infants who died compared with asphyxiated survivors (11.64±1.31 vs 6.18±2.48, P=0.0004).
Conclusion:
LPO was increased and correlated with severity of asphyxia as well as with mortality. Further studies are warranted to examine whether it is only a marker for outcome or a contributor in the pathogenesis of hypoxic–ischemic brain injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stoll BJ, Kliegman RM. Hypoxia-ischemia. In: Behrman RE, Kliegman RM, Jenson HB (eds). Nelson Textbook of Pediatrics, 18th edn. Saunders: Philadelphia, 2007, pp 566–568.
Thornberg E, Thiringer K, Odeback A, Milsom I . Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr 1995; 84: 927–932.
Orrock JE, Panchapakesan K, Vezina G, Chang T, Harris K, Wang Y et al. Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy. Pediatr Res 2016; 79 (5): 742–747.
Aly H, Khashaba MT, El-Ayouty M, El-Sayed O, Hasanein BM . IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev 2006; 28: 178–182.
Aly H, Khashaba MT, Nada A, Hasanen BM, McCarter R, Schultz SJ et al. The role of complement in neurodevelopmental impairment following neonatal hypoxic-ischemic encephalopathy. Am J Perinatol 2009; 26: 659–665.
Khashaba MT, Shouman BO, Shaltout AA, Al-Marsafawy HM, Abdel-Aziz MM, Patel K et al. Excitatory amino acids and magnesium sulfate in neonatal asphyxia. Brain Dev 2006; 28: 375–379.
Buonocore G, Groenendaal F . Antioxidant strategies. Semin Fetal Neonatal Med 2007; 12: 287–295.
Duan C, Yan F, Song X, Lu GW . Changes of superoxide dismutase, glutathione perioxidase and lipid peroxides in the brain of mice preconditioned by hypoxia. Biol Signals Recept 1999; 8: 256–260.
Glistrap LC 3rd, Levono KJ, Burris J, Williams ML, Little BB . Diagnosis of birth asphyxia on the basis of fetal pH, Apgar score and newborn cerebral dysfunction. Am J Gynecol Obstet 1989; 161: 825–830.
Chumakov MV, Efremov AA, NIu Zvereva, Dublev AV, Davydov BV, IaB Brand et al. Effects of anesthetics on oxidative stress changes in patients with high anesthetic risk in the perioperative period of coronary bypass surgery. Anesteziol Reanimatol 2008; 4: 5–8.
Cinnella G, Vendemiale G, Dambrosio M, Serviddio G, Pugliese PL, Aspromonte G et al. Effect of propofol, sevoflurane and desflurane on systemic redox balance. Int J Immunopathol Pharmacol 2007; 20 (3): 585–593.
Florek E, Ignatowicz E, Nowakowska A, Piekoszewski W, Kulza M, Saija A et al. Effect of combined exposure to ethanol and tobacco smoke on lipid peroxidation in rats. Przegl Lek 2009; 66: 655–659.
Sarnat HB, Sarnat MS . Neonatal encephalopathy following fetal distress. Arch Neurol 1976; 33: 696–705.
Roomi MW, Hopkins CY . Some reactions of sterculic and malvalic acids. A new source of malvalic acid. Can J Biochem 1970; 48: 759–762.
Shouman BO, Mesbah A, Aly H . Iron metabolism and lipid peroxidation products in infants with hypoxic ischemic encephalopathy. J Perinatol 2008; 28: 487–491.
Nagdyman N, Kömen W, Ko HK, Müller C, Obladen M . Early biochemical indicators of hypoxic-ischemic encephalopathy after birth asphyxia. Pediatr Res 2001; 49: 502–506.
Gazzolo D, Abella R, Marinoni E et al. New markers of neonatal neurology. J Matern Fetal Neonatal Med 2009; 22 (Suppl 3): 57–61.
Beken S, Aydin B, Dilli D, Erol S, Zenciroğlu A, Okumuş N . Can biochemical markers predict the severity of hypoxic-ischemic encephalopathy? Turk J Pediatr 2014; 56: 62–68.
Boskabadi H, Maamouri G, Sadeghian MH, Ghayour-Mobarhan M, Heidarzade M, Shakeri MT et al. Early diagnosis of perinatal asphyxia by nucleated red blood cell count: a case-control study. Arch Iran Med 2010; 13: 275–281.
Barrera-de León JC, Cervantes-Munguía R, Vásquez C, Higareda-Almaraz MA, Bravo-Cuellar A, González-López L . Usefulness of serum lipid peroxide as a diagnostic test for hypoxic ischemic encephalopathy in the full-term neonate. J Perinatol 2013; 33: 15–20.
Ogihara T, Hirano K, Ogihara H . Non-protein-bound transition metals and hydroxyl radical generation in cerebrospinal fluid of newborn infants with hypoxic ischemic encephalopathy. Pediatr Res 2003; 53: 594–599.
Mir IN, Chalak LF . Serum biomarkers to evaluate the integrity of the neurovascular unit. Early Hum Dev 2014; 90: 707–711.
Erisir M, Kandemir FM, Yuksel M . The effects of Caesarean section on lipid peroxidation and some antioxidants in the blood of newborn calves. Vet Arhiv 2013; 83: 153–159.
Rogers MS, Mongelli JM, Tsang KH, Wang CC, Law KP . Lipid peroxidation in cord blood at birth: the effect of labour. Br J Obstet Gynaecol 1998; 105: 739–744.
Mohamed MA, Aly H . Impact of race on male predisposition to birth asphyxia. J Perinatol 2014; 34: 449–452.
Aly H, El Beshlawy A, Badrawi N, Mohsen L, Mansour E, Ramy N et al. Thrombopoietin level is increased in the serum of asphyxiated neonates: a prospective controlled study. J Perinatol 2005; 25: 320–324.
Smith AL, Garbus H, Rosenkrantz TS, Fitch RH . Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci 2015; 5: 220–240.
Acknowledgements
The author thanks Veronica Amaya at George Washington University for help editing the manuscript and the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramy, N., Al Sharany, W., Mohamed, M. et al. Lipid peroxides in the serum of asphyxiated neonates. J Perinatol 36, 849–852 (2016). https://doi.org/10.1038/jp.2016.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.97