Abstract
Objective:
To compare the incidence of metabolic acidosis and feeding intolerance associated with powdered or acidified liquid human milk fortifier (HMF).
Study Design:
This retrospective study evaluated infants ⩽32 weeks gestational age or ⩽1500 g birth weight who received human milk with either powdered or acidified liquid HMF (50 consecutively born infants per group). Primary outcomes tracked were metabolic acidosis (base excess less than −4 mmol l−1 or bicarbonate less than 18 mmol l−1), feeding intolerance (gastric residual >50% feed volume, >3 loose stools or emesis per day, abdominal tenderness or distention), necrotizing enterocolitis, late-onset infection, death, length of hospital stay and ability to remain on HMF. Demographics, feeding practices, growth parameters and laboratory data were also collected.
Result:
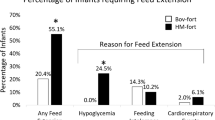
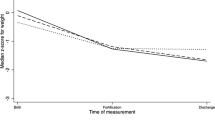
Significantly more infants who received acidified liquid HMF developed metabolic acidosis (P<0.001). Base excess and bicarbonate were both significantly decreased after HMF addition in the liquid HMF group (base excess P=0.006, bicarbonate P<0.001). More infants were switched off liquid HMF due to metabolic acidosis or feeding intolerance than those on powdered HMF (P<0.001). Despite increased protein intake in the liquid HMF group (P=0.009), both groups had similar enteral caloric intakes with no difference in growth rates between the two groups. There was no significant difference in any of the other primary outcomes.
Conclusion:
Infants receiving acidified liquid human milk fortifier were more likely to develop metabolic acidosis and to be switched off HMF than those who received powdered HMF. Growth in the liquid HMF group was no different than the powdered group, despite higher protein intake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
American Academy of Pediatrics Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics 2005; 115: 496–506.
Hylander MA, Strobino DM, Dhanireddy R . Human milk feedings and infection among very low birth weight infants. Pediatrics 1998; 102: E38.
Schanler RJ, Shulman RJ, Lau C . Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999; 103 (6): 1150–1157.
Narayanan I, Murthy NS, Prakash K, Gurjal VV . Randomised controlled trial of raw and Holder pasteurized human milk and of formula supplements on incidence of neonatal infection. Lancet 1984; 8412: 1111–1113.
Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM . Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007; 27: 428–433.
Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF for the National Institute of Child Health and Human Development Neonatal Research Network. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 2009; 29: 57–62.
Lucas A, Cole TJ . Breast milk and neonatal necrotising enterocolitis. Lancet 1990; 336: 1519–1523.
Lucchini R, Bizzarri B, Giampietro S, DeCurtis M . Feeding intolerance in preterm infants: how to understand the warning signs. J Matern Fetal Neonatal Med 2011; 24 (S1): 72–74.
Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006; 118 (1): e115–e123.
Horwood LJ, Darlow BA, Mogridge N . Breast milk feeding and cognitive ability at 7-8 years. ArchDis Child Fetal Neonatal Ed 2001; 84: F23–F27.
Schanler RJ . The use of human milk for premature infants. Pediatr Clin N Amer 2001; 48: 207–219.
Kashyap S, Schulze KF, Forsyth M, Dell RB, Ramakrishnan R, Heird WC . Growth, nutrient retention, and metabolic response of low-birth-weight infants fed supplemented and unsupplemented preterm human milk. Am J Clin Nutr 1990; 52: 254–262.
Wojcik KY, Rechtman DJ, Lee ML, Montoya A, Medo ET . Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc 2009; 109: 137–140.
Maayan-Metzger A, Avivi S, Schushan-Eisen I, Kuint J . Human milk versus formula feeding among preterm infants: short-term outcomes. Am J Perinatol 2012; 29: 121–126.
Centers for Disease Control and Prevention. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. MMWR 2002; 51 (14): 298–300.
American Academy of Pediatrics. CDC, FDA advise against powdered formula in NICUs. AAP News 2002; 20: 219.
Kim Hae-Young . Statistical notes for clinical researchers: assessing normal distribution using skewness and kurtosis. Restor Dent Endod 2013; 38 (1): 52–54.
Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European society for paediatric gastroenterology, hepatology, and nutrition committee on nutrition. JPGN 2010; 50 (1): 85–91.
Abbott Nutrition, Abbott Laboratories. Similac Human Milk Fortifier—powdered unflavored (nutritional information on the internet, updated 12 December 2012). Available at http://abbottnutrition.com/brands/products/similac-human-milk-fortifier (last accessed 16 May 2014).
Mead Johnson & Company, LLC. Enfamil Human Milk Fortifier Acidified Liquid—Enfamil (nutritional information on the internet, updated 20 October 2011. Available at http://www.enfamil.com/app/iwp/enf12/product.do?dm=enf&id=/Consumer_Home3/FeedingSolutions/EnfamilHumanMilkFortifier2&iwpst=B2C&ls=0&csred=1&r=3552909950 (last accessed 18 May 2012).
Moya F, Sisk PM, Walsh KR, Berseth CL . A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics 2012; 130 (4): e928–e935.
Erickson T, Gill G, Chan GM . The effects of acidification on human milk’s cellular and nutritional content. J Perinatol 2013; 33: 371–373.
Thoene M, Hanson C, Lyden E, Dugick L, Ruybal L, Anderson-Berry A . Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014; 6: 261–275.
Green J, Maor G . Effect of metabolic acidosis on the growth hormone/IGF-1 endocrine axis in skeletal growth centers. Kidney Int 2000; 57 (6): 2258–2267.
McSherry E, Morris RC Jr . Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest 1978; 61: 509–527.
Rochow N, Jochum F, Redlich A, Korinekova Z, Linnemann K, Boehm G et al. Fortification of breast milk in VLBW infants: metabolic acidosis is linked to the composition of fortifiers and alters weight gain and bone mineralization. Clin Nutr 2011; 30: 99–105.
Kalhoff H, Manz F . Nutrition, acid-base status and growth in early childhood. Eur J Nutr 2001; 40 (5): 221–230.
Kalhoff H, Manz F, Diekmann L, Kunz C, Stock GJ, Weisser F . Decreased growth rate of low-birthweight infants with prolonged maximum renal acid stimulation. Acta Paediatr 1993; 82: 522–527.
Senger H, Boehm G, Beyreiss K, Braun W . Cholestasis in late metabolic acidosis of prematurely born infants. J Clin Chem Clin Biochem 1987; 25 (7): 413–418.
Roberts D, Dalziel SR . Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006; ((3)) CD004454.
Acknowledgements
We gratefully acknowledge the assistance of Melissa Bowles, MSN, RN, PNP-BC, in collecting data. We also thank the staff involved in the maintenance of the neonatal database, Linda Breuer, LPN and Phyllis Anderson, RT and dietitian Rita Chrivia, RD, CSP, LD for assistance with calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cibulskis, C., Armbrecht, E. Association of metabolic acidosis with bovine milk-based human milk fortifiers. J Perinatol 35, 115–119 (2015). https://doi.org/10.1038/jp.2014.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.143
This article is cited by
-
Human milk pH is associated with fortification, postpartum day, and maternal dietary intake in preterm mother-infant dyads
Journal of Perinatology (2023)
-
The effects of human milk fortification on nutrients and milk properties
Journal of Perinatology (2017)
-
Reply to ‘Breast milk: the best lovebiotic’
Journal of Perinatology (2015)
-
Breast milk: the best lovebiotic
Journal of Perinatology (2015)