Abstract
Objective:
Postnatal steroids are used in neonatal intensive care units despite known side effects. Hydrocortisone (HC) use persists as it is believed to have less deleterious effects on neurodevelopmental (ND) outcome compared to other steroids. The literature is sparse with respect to the ND impact of HC use in recent years. Hence, we sought to examine the effect of HC use on ND outcome in a contemporary cohort of extremely low birth weight (ELBW) infants.
Study Design:
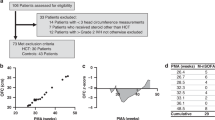
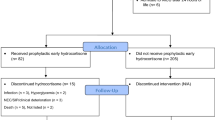
A total of 175 ELBW infants (86 HC exposed, 89 steroid naive) born in 2008 to 2010 were compared for mortality, morbidity and ND outcome at 8 and 20 months corrected age. Outcome measures included neurologic exam and results of the Bayley Scales of Infant and Toddler Development-III (BSITD-III). Multiple regression analyses adjusted for the effect of other risk factors on outcome.
Result:
Overall, 65 (75%) of the HC and 74 (83%) of the no-HC groups survived to discharge. HC infants were smaller (mean birth weight (BW) 719±127 g vs 837±99 g) and of lower gestational age (GA) (mean GA 26.0±1.7 weeks vs 27.5±1.8 weeks) compared to the no-HC group. Patients in the HC group were more likely to be a multiple, have a severely abnormal head ultrasound, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis and receive treatment for patent ductus arteriosus and hypotension than those in the no-HC group. Of the HC group, the mean age at treatment was 20±19 days, mean duration of treatment 49±37 days. At 8 months, the HC group had lower mean motor (87±18 vs 95±15, P=0.028) and fine motor (9±2.9 vs 10.5±2.6, P=0.005) and higher rate of subnormal motor (44 vs 15%, P=0.002) and fine motor scores (24 vs 6.5%, P=0.017). In regression analyses, HC exposure >7 days was significantly related to worse outcome on fine motor scores at 8 months while cumulative days of HC exposure was a predictor of worse outcome on language at 8 months and motor outcome at 20 months. Each additional day of HC exposure increased the odds of subnormal receptive and expressive language in the first year of life by 4 and 2%, respectively, and increased odds of subnormal motor function by 2% in the 2nd year of life.
Conclusion:
HC exposure for >7 days is associated with worse performance in fine motor skills in the first year of life, while cumulative HC exposure negatively impacts receptive and expressive language skills in the first year and motor skills in the second year of life after adjusting for neonatal and social risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 1998; 101 (5): E7.
O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 1999; 104: 15–21.
Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2000; 83: F177–F181.
Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 2004; 350 (13): 1304–1313.
Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009; 123: e430–e437.
Barrington KJ . The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 2001; 1–1.
Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002; 109: 330–338.
American Academy of Pediatrics. Policy statement: postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010; 126 (4): 800–808.
Yoder BA, Harrison M, Clark RH . Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009; 124: 673–679.
Fortin-Pellerin É, Petersen C, Lefebvre F, Barrington KJ, Janvier A . Evolving neonatal steroid prescription habits and patient outcomes. Acta Paediatrica 2013; 102: 799–804.
Van der Heide-Jalving M, Kamphuis P, van der Laan M, Bakker J, Wiegant V, Heijnen C et al. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatrica 2003; 92: 827–835.
Rademaker KJ, Uiterwaal CS, Groenendaal F, Venema MM, van Bel F, Beek FJ et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr 2007; 150: 351–357.
Rademaker KJ, de Vries LS, Uiterwaal CS, Groenendaal F, Grobbee DE, van Bel F . Hydrocortisone treatment for chronic lung disease in the preterm newborn and long-term neurodevelopmental follow-up. Arch Dis Child Fetal Neonatal Ed 2008; 93 (1): F58–F63.
Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA et al. Neonatal Hydrocortisone Working Group. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology 2009; 95 (3): 240–247.
Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007; 120: 40–48.
Fenton T . A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and new format. BMC Pediatr 2003; 3: 13.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1979; 187 (1): 1–7.
Bayley N . Bayley Scales of Infant DevelopmentThird ednSan Antonio, TX: The Psychological Corporation, 2006.
Amiel-Tison C, Stewart AL . Follow-up studies in the first five years of life: a pervasive assessment of neurologic function. Arch Dis Child 1989; 64: 496–502.
Needelman H, Hoskoppal A, Roberts H, Evans M, Bodensteiner JB . The effect of hydrocortisone on neurodevelopmental outcome in premature infants less than 29 weeks’ gestation. J Child Neurol 2010; 25: 448–452.
Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr 2012; 161 (2): 222–228.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Patra, K., Greene, M. & Silvestri, J. Neurodevelopmental impact of hydrocortisone exposure in extremely low birth weight infants: outcomes at 1 and 2 years. J Perinatol 35, 77–81 (2015). https://doi.org/10.1038/jp.2014.133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.133
This article is cited by
-
Duration of mechanical ventilation is more critical for brain growth than postnatal hydrocortisone in extremely preterm infants
European Journal of Pediatrics (2021)