Abstract
Objective:
Pulse oximetry has been recognized as a promising screening tool for critical congenital heart disease (CCHD). The aim of this research was to study the feasibility of implementation in a community hospital setting.
Study Design:
Meetings were conducted to determine an implementation plan. Pulse oximetry was performed on the right hand and foot after 24 h of age. Newborns with a saturation ⩽95% or a ⩾3% difference were considered to have a positive screen. Screening barriers, screening time and ability to effectively screen all eligible newborns were noted.
Result:
From January 2009 through May 2010, of 6841 eligible newborns, 6745 newborns (98.6%) were screened. Of the nine infants with positive pulse oximetry screens, one had CCHD, four had CHD and four others were determined to have false positive screens. Average screening time was 3.5 min (0 to 35 min).
Conclusion:
Pulse oximetry can be implemented successfully in community hospitals without an excessive number of false positives or additional nursing staff.
Similar content being viewed by others
Introduction
Congenital heart disease (CHD) is the most common birth defect and affects approximately 8 per every 1000 newborns born each year. Critical CHD (CCHD), severe types of CHD, has an incidence of approximately 2.5 to 3 per 1000 live births.1 These more serious defects cause significant morbidity and mortality, accounting for nearly 40% of deaths in children with congenital anomalies in the first year of life.2 Over the past two decades numerous advances in care have resulted in a significant reduction in mortality secondary to CCHD; however, timely diagnosis remains an issue for these newborns. Despite prenatal diagnosis and newborn examinations, as many as 39% of infants diagnosed with CCHD are diagnosed only after discharge from the newborn nursery.3 Delay in diagnosis may have significant adverse implications; one study showed that 43% of cases diagnosed after hospital discharge from the nursery were in shock at the time of readmission.4
Pulse oximetry has been recommended as a potential newborn screening test for CCHD. Early efforts provided the conceptual basis for pulse oximetry in the detection of CCHD.5, 6, 7, 8, 9 Subsequent work has provided additional evaluation of the sensitivity, specificity and diagnostic gap of pulse oximetry screening.10, 11, 12, 13, 14 In 2009, the American Heart Association (AHA) and American Academy of Pediatrics (AAP) released a statement on the potential use of pulse oximetry screening to detect CCHD.15 The statement recognized that the most favorable outcomes are realized when screening on the right lower extremity is conducted after 24 h of age, using 95% as the cutoff value for additional consultation and evaluation. The AHA and AAP concluded that pulse oximetry screening could potentially improve detection of CCHD. However, universal screening was not endorsed at the time and the authors recommended that ‘future studies in larger populations and across a broad range of newborn delivery systems are needed to determine whether this practice should become standard of care in the routine assessment of the neonate.’
The primary aims of this research were to determine if pulse oximetry screening for the detection of CCHD could be successfully implemented in a large community hospital and to evaluate the feasibility of implementation (ability to screen all participants, obstacles encountered during screening and impact on staffing and/or work flow). A secondary aim of the study was to determine the number of participants with CCHD identified by suggested program design.
Methods
A prospective study of implementation was performed at Holy Cross Hospital (HCH), a large community hospital with approximately 8500 deliveries per year in Silver Spring, MD, USA. The study period was from January 2009 through May 2010. The study population consisted of newborns being admitted to the well baby nursery; mothers of newborns admitted to the neonatal intensive care unit (NICU) were not approached for participation. Mothers whose newborns were eligible for participation were informed about the study through an educational information sheet (in English and Spanish) and provided verbal consent if they wished to participate. The enrolled newborns meeting the following criteria were eligible for pulse oximetry screening: term or late preterm (⩾35 weeks gestation), no prenatal diagnosis of CHD, no dysmorphic features and no signs of cardiovascular abnormalities, such as cyanosis, abnormal vital signs or a cardiac murmur. The institutional review boards (IRB) of Children's National Medical Center (CNMC) and HCH approved the study.
Implementation planning
During the development of the research protocol, investigators from CNMC worked with hospital and nursery leadership from HCH to determine the best practice for adding pulse oximetry screening to routine newborn care. Individuals from HCH included the research team (the nursing director of perinatal services, the medical director of NICU and the physician pediatric education director), the Chief Nursing Officer, assistant nurse managers of the maternity suites, the unit educators and the nursing staff (registered nurses, licensed practical nurses and certified nursing assistants) who would be involved in research efforts and screening of newborns. The final protocol design was formulated through these collaborative meetings.
Training and education
Prior to the implementation of screening, all members of the nursing staff practicing in the maternity suites and labor and delivery were required to attend an educational in-service, lasting approximately 1 h. Training was conducted over 2 weeks covering all shifts to ensure that all providers received the training. Each in-service included education on protocol aims, background and significance of pulse oximetry screening for CCHD, eligibility requirements for newborns, study design and methods, potential risks and benefits, and confidentiality requirements for research participants. The staff responsible for conducting screening completed hands-on training on correct pulse oximetry technique, conducted by a representative from the manufacturer, Masimo Corporation (Irvine, CA, USA). Completion of a competency checklist and a knowledge assessment quiz was required for all the nursing staff. In-service trainings were provided accordingly for newly hired staff to ensure that 100% of staff was educated. In addition, each member of the nursing staff, ultrasonographer, licensed independent practitioner (LIP) and physician responsible for providing care in labor and delivery, newborn nursery, maternity suites and NICU received a letter of study intent from the investigators.
Implementation of screening
Pulse oximetry screening was performed in conjunction with newborn metabolic screening. One certified nursing assistant was assigned to perform metabolic and pulse oximetry screening of each eligible newborn. When that individual was not scheduled to work, another nursing staff member was assigned newborn pulse oximetry screens. Pulse oximetry of the right hand and the right foot was conducted between 24 h of age and discharge from the nursery. The Masimo Radical-7 pulse oximeter and a disposable low noise cable sensor were used to screen each newborn. Disposable sensors were provided by the study grant and used to avoid concerns regarding potential for transmission of infection with reusable probes. To ensure accuracy of the reading obtained, the individual responsible for screening verified all confidence indicators, including the signal identification quality and perfusion index, before reporting saturations. A time requirement for performing screening on each extremity was not specified, since confidence indicators were used to indicate readings as accurate. The individual responsible for screening recorded age (in hours) of newborn, time that pulse oximetry screening began and ended, obstacles encountered with equipment, newborn, family or staff and time spent overcoming obstacles.
If the oxygen saturation was >95% for both the right hand and the right foot and there was <3% difference between the two, the test was considered negative and the newborn ‘passed’ screening; no further cardiac evaluation in the well baby nursery was necessary unless indicated by subsequent physical exam or clinical condition. If the oxygen saturation was ⩽95% for any measurement or if there was ⩾3% difference between the two saturations, the test was considered positive and the newborn was ‘referred’ to his or her physician or LIP. The newborn's physician or LIP was informed and responsible for all future decisions regarding care and evaluation. For newborns who were ‘referred’, it was recommended that echocardiography be obtained to evaluate cardiac anatomy and if the oxygen saturation was ⩽90% that he or she be transferred to the NICU for further monitoring and evaluation. Decisions regarding echocardiography, additional consultation or transfer to the NICU were made at the discretion of the physician or LIP caring for the newborn (Figure 1).
Results
During enrollment, 6860 mothers agreed to have their newborn participate in the study (Figure 2). Of these, 6841 newborns met eligibility requirements for screening and 6745 newborns received full screening (defined as pulse oximetry of both the right hand and right foot). A total of 19 newborns did not meet eligibility criteria: 15 demonstrated signs or symptoms of CHD, 3 had dysmorphic features and 1 newborn was ineligible for reasons not indicated. No further follow-up of these 19 newborns was obtained according to the protocol, and therefore it is unknown if they were identified to have CCHD. In addition, 96 eligible newborns were not screened because of staff workload, obstacles faced while performing screening or early discharge.
The average age at which screening occurred was 42.4 h (21 to 98 h). The average upper extremity oxygen saturation was 100% (93 to 100%). The average lower extremity oxygen saturation was 100% (90 to 100%). The average difference between upper and lower extremity oxygen saturations was 0.2% (0 to 6%). During the study period, of the 6745 newborns screened, 9 had positive screens and were referred to their primary physician or LIP for decision regarding additional testing. Five additional newborns were incorrectly identified as having negative screens; all of these newborns had saturations above 95% but had a difference of 3% between the right hand and right foot; none of these infants had additional testing or consultation. Of the 9 newborns that were referred, 6 had additional testing or consultation, including repeat pulse oximetry (1), echocardiograms (6) and cardiology consultation (5). Transfer to the NICU was required for two infants, one with CCHD and one with mirror image dextrocardia and lobar pneumonia.
Five infants were identified with CHD. One of these infants had oxygen saturations of 93 and 91%, and was subsequently diagnosed with CCHD (anomalous drainage of the superior vena cava to left atrium). Four others were diagnosed with CHD (dextrocardia, dilated ascending aorta and two atrial septal defects). One infant with oxygen saturations of 100 and 97% was found upon further evaluation to have no CHD and was confirmed as a false positive. Three other infants had referring saturations but they had no additional testing or consultation ordered. Assuming that the three infants with positive screens and no additional workup did not have CHD, there were four referred infants with false positive results.
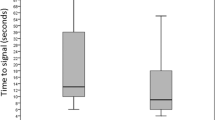
The average amount of time required for screening was 3.5 min (0 to 35 min). Barriers to performing screening were identified for a total of 166 of the 6841 enrolled newborns eligible for screening (2.4%). Barriers were identified in 97 of 6745 infants who were successfully screened (1.4%) compared with 69 of 96 infants not screened (71.9%). Barriers were identified to be related to the screening equipment (53.6% of the time) (for example, ‘machine didn’t work’ or ‘difficulty with placement’), staff (22.9%) (for example, ‘busy day’ or ‘too many metabolic screens’), infant (19.9%) (for example, ‘infant crying’ or ‘very active’) and family (3.6%) (for example, ‘mom was in rush’). In 100% of cases where the barrier was staff-related, the infant was ultimately not screened. Time required to overcome barriers was documented in 34 infants successfully screened and averaged 5.1 min per infant (2 to 10 min).
Discussion
Our findings demonstrate that implementation is feasible and does not result in an excessive number of false positives. It was particularly important for feasibility to be assessed in a community hospital, as the vast majority of infants in the United States are born in such settings. In contrast, most pulse oximetry screening studies have been performed in research centers that have access to additional resources.
In August 2011, a national workgroup commissioned by the United States Health and Human Services (HHS) Secretary's Advisory Committee on Heritable Diseases in Newborns and Children published strategies to implement newborn pulse oximetry screening.16 The following month, HHS Secretary Kathleen Sebelius fully endorsed adding screening to the recommended uniform screening panel and directed national agencies ‘to proceed with implementation expeditiously’.17 Given these national endorsements, universal pulse oximetry screening will become standard of care after the development of needed infrastructure. Our study supports the feasibility of implementation in a community hospital setting and evaluates screening ‘in larger populations and across a broad range of delivery systems’,15 as requested by the AHA and AAP in 2009.
In our view, implementation feasibility means a high success rate of screening along with sufficiently low barriers and resource drain. Successful screening occurred in the large majority of eligible participants (98.6%). We speculate that assigning that task to a single certified nursing assistant who was responsible for the screening whenever she was working allowed for more consistent and efficient care leading to a high success rate of screening. Barriers to screening occurred for a variety of reasons but at a very low frequency (2.4% overall). Resource equipment utilization includes the pulse oximeter, which is standard equipment in most hospitals, and the probes needed to perform screening. Since the research study reported here, HCH has continued newborn pulse oximetry screening as standard of care but transitioned to reusable probes to minimize cost.
Pulse oximetry screening can be implemented without requiring additional nursing staff. Existing staff from labor and delivery and the maternity suites provided education to the providers and parents and performed all screening protocols. Taking advantage of staffing models already in place, the program design linked pulse oximetry screening with newborn metabolic screening so that a small, consistent group of the nursing staff held primary responsibility for ensuring that both screenings were completed before discharge. Pulse oximetry screening time was reported to be <4 min per newborn on average.
Inclusion of pulse oximetry screening in newborn care did not necessarily result in a strain on pediatricians, neonatologists or ultrasonographers due to additional evaluations of failed screens. During the entire 17-month research period, only 9 of the 6745 newborns screened were referred to their pediatrician for additional evaluation. In addition, six echocardiograms were ordered for these newborns with positive pulse oximetry screens. Historical data shows that 222 echocardiograms were ordered in the well baby nursery for other indications during that same time period.
To date, few studies in the United States have evaluated pulse oximetry screening in non-research settings. Walsh presented data from a public health initiative in 2007 offering pulse oximetry screening to all institutions that delivered newborns in Middle Tennessee. Seventy-seven percent of hospitals agreed to participate, representing 43% of all births occurring in the region during the study period. The largest center with 7000 deliveries, among others, chose not to participate due to concerns about the need for extra staffing. Of the 14 983 infants offered screening, 97.2% were successfully screened, almost as high as in our study. Additionally, 0.78% of all infants screened failed testing compared with 0.12% in our study. Similar to our study, one patient with CCHD was ultimately identified. Many of the hospitals in the Tennessee study did not have access to pediatric cardiology services on site and as such only 2.65% (3 of 113) of failed screens had echocardiograms performed, compared with our 67% (6 of 9).18
The design and results of our study and Walsh's differ. We suspect these differences may be partially explained by education and training, nursery care delivery systems and available resources. Walsh's data came from a public health initiative, without funding for education and training beyond set-up of the pulse oximeter. With additional training and defined confidence indicators for reporting accurate readings, it is not surprising that our screening took slightly longer on average but resulted in fewer failed screens. The educational resources developed to train staff and educate parents during our study have led to the development of an evidence-based educational toolkit.19 Efforts are currently under way to evaluate this toolkit in providing aid to nurseries interested in implementing pulse oximetry screening.
Another major difference lies in HCH's ability to obtain an echocardiogram and cardiology consult quickly owing to an affiliation with a nearby academic center. This led to a very different approach to pulse oximetry screening and referral patterns in our institution. We recognize this to be a limitation of our study in that our results may not be generalizable to nurseries that do not have echocardiography on site, and further evaluation would require access to telemedicine or transfer to another institution. Future studies and public health agencies will need to address this circumstance to maximize access to care.
After completion of our research and successful implementation of the newborn pulse oximetry screening program, analysis and discussion identified a factor not formally studied. Physician and nursing ‘champions’ for screening were noted to be important during the implementation process. Two HCH-based physician investigators were on site, the CNMC-based nursing research coordinator made intermittent visits, and over time staff nursing champions were identified due to interest shown. These champions ensured that screening policy and protocols were carried out appropriately by serving as advocates for screening, being available for onsite support and clarification of protocols, encouraging staff during daily operations, and periodically presenting educational reinforcement and updated research findings. We speculate that these champions were instrumental in the success of implementation.
Regarding our secondary aim, we note that our study found a low incidence of CCHD identified by pulse oximetry screening. The true incidence of CCHD in the population delivered at HCH during the study period could not be determined due to IRB constraints and enrollment and eligibility requirements. Because this was a feasibility study, only verbal consent was obtained, and mothers were informed that contact would not be initiated following discharge. Consequently, the research team was unable to determine false negatives for CCHD. However, the authors of this paper (including a neonatologist at HCH and two cardiologists at CNMC, the primary referral center for pediatric cardiology in the region) were unaware of any subsequent diagnoses of CCHD during the research period. Therefore, it is probable that there were no false negatives.
The low number of CCHD patients identified among screened participants can be partially explained by defined research exclusion criteria causing preselection bias. First, no patients admitted to the NICU were included in the study; the NICU population disproportionally includes infants with clinical presentations frequently associated with CCHD (respiratory distress, hemodynamic instability or with multiple congenital anomalies). Second, HCH has a strong maternal fetal medicine program with active fetal echocardiography that may result in a higher percentage of CHD diagnosed prenatally than is typical in a community hospital; all newborns with prenatally diagnosed CHD met exclusion criteria. Finally, 15 neonates in the well baby nursery were ineligible to be screened due to specific clinical concern for CHD; some of these patients may have actually had CCHD but we do not know as no follow-up was obtained on these patients per study protocol.
Conclusions
Newborn pulse oximetry screening for CCHD is feasible and can be successfully implemented in a community hospital setting with limited resource drain (including no increase in staffing), few barriers to screening and a low false-positive rate. Given recent national endorsements to move forward with universal pulse oximetry screening, these results are particularly important for other community hospital settings. Although not formally studied, subsequent discussions suggest that careful implementation planning, appropriate education and training, and the support of stakeholders and champions may be important factors when implementing similar screening programs elsewhere.
References
Hoffman JIE, Kaplan S . The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39: 890–1900.
Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD . Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979-1997. Circulation 2001; 103: 2376–2381.
Acharya G, Sitras V, Maltau JM, Dahl LB, Kaaresen PI, Hanssen TA et al. Major congenital heart disease in Northern Norway: shortcomings of pre- and postnatal diagnosis. Acta Obstet Gynecol Scand 2004; 83: 1124–1129.
Mellander M, Sunnegårdh J . Failure to diagnose critical heart malformations in newborns before discharge-an increasing problem? Acta Paediatrica 2006; 95: 407–413.
Koppel RI, Druschel CM, Carter T, Goldberg BE, Mehta PN, Talwar R et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics 2003; 111 (3): 451–455.
Valmari P . Should pulse oximetry be used to screen for congenital heart disease? Arch Dis Child Fetal Neonatal Ed 2007; 92: F219–F224.
Hoke TR, Donohue PK, Bawa PK, Mitchell RD, Pathak A, Rowe PC et al. Oxygen saturation as a screening test for critical congenital heart disease; a preliminary study. Pediatr Cardiol 2002; 23 (4): 403–409.
Meberg A, Brugmann-Pieper S, Due R, Eskedal L, Fagerli I, Farstad T et al. First day of life pulse oximetry screening to detect congenital heart Defects. J Pediatr 2008; 152 (6): 761–765.
Granelli AD, Mellander M, Sunnegardh J, Sandberg K, Ostman-Smith I . Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta Paediatrica 2005; 94: 1590–1596.
Granelli AD, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganas L et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish Prospective Screening Study in 39,821 newborns. BMJ 2008; 337: a3037.
Riede FT, Worner C, Dahnert I, Mockel A, Kostelka M, Schneider P . Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine – results from a prospective multicenter study. Eur J Pediatr 2010; 169: 975–981.
Thangaratinam S, Daniels J, Ewer AK, Zamora J, Khan KS . Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: a systematic review. Arch Dis Child Fetal Neonatal Ed 2007; 92: F176–F180.
Hokanson JS . Pulse oximetry screening for unrecognized congenital heart disease in neonates. Congenit Cardiol Today 2011; 9 (1): 1–6.
Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S et al. Pulse oximetry screening for congenital heart defects in newborn infants (Pulseox): a test accuracy study. Lancet 2011; 378 (9793): 785–794.
Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Pediatrics 2009; 124: 823–836.
Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow RW et al. Strategies for implementing screening for critical congenital heart disease: recommendations of the United States Health and Human Services Secretary's Advisory Committee on Heritable Disorders in Newborns and Children. Pediatrics 2011; 128 (5): e1259–e1267.
Sebelius K . Letter to R Rodney Howell, MD (Internet), 2011 (updated 21 September 2011; cited 23 September 2011). Available from: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/.
Walsh W . Evaluation of pulse oximetry screening in Middle Tennessee: cases for consideration before universal screening. J Perinatol 2011; 31: 125–129.
Children's National Medical Center. Congenital Heart Disease Screening Program Toolkit: A Toolkit for Implementing Screening. Children's National Medical Center: Washington, DC, 2009.
Acknowledgements
Holy Cross Hospital Departments of Pediatrics, Nursing and Patient Care Services, Elsie and Marvin Dekelboum Family Foundation. This work was funded by a gift from The Elsie and Marvin Dekelboum Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bradshaw, E., Cuzzi, S., Kiernan, S. et al. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol 32, 710–715 (2012). https://doi.org/10.1038/jp.2011.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.179
Keywords
This article is cited by
-
Feasibility of screening for critical congenital heart disease using pulse oximetry in Indonesia
BMC Pediatrics (2022)
-
Screening for Critical Congenital Heart Disease: A Matter of Sensitivity
Pediatric Cardiology (2013)
-
Feasibility of Pulse Oximetry Screening for Critical Congenital Heart Disease at 2643-Foot Elevation
Pediatric Cardiology (2013)