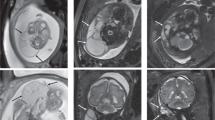
Abstract
We report a case of myelomeningocele in an infant whose mother was exposed to efavirenz during the first 16 weeks of pregnancy. Although the true risk for myelomeningocele with the use of efavirenz early in pregnancy is still unknown, the findings in humans are consistent with those observed in primates and suggest that efavirenz is a potent teratogen. Thus, we suggest that efavirenz only be prescribed for women of childbearing potential when no other comparable antiretroviral options are available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004;350:1850–1861.
Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med 1999;341:1865–1873.
Perinatal HIV Guidelines Working Group. Public Health Service Task Force. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States Available on at: http://www.aidsinfo.nih.gov/guidelinesupdated June 23, 2004. Accessed January 6, 2005.
Nightingale SL . From the food and drug administration. JAMA 1998;280:1472.
Fundaro C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E . Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS 2002;16:299–300.
De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G . Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med 2002;162:355.
Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for January 1989 through 31 January 2004. Wilmington, NC: Registry Coordinating Center; 2004. Available from URL: www.apregistry.com.
Botto LD, Moore CA, Khoury MJ, Erickson JD . Neural-tube defects. N Engl J Med 1999;341:1509–1519.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saitoh, A., Hull, A., Franklin, P. et al. Myelomeningocele in an Infant with Intrauterine Exposure to Efavirenz. J Perinatol 25, 555–556 (2005). https://doi.org/10.1038/sj.jp.7211343
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211343
This article is cited by
-
Evaluating Neurodevelopmental Consequences of Perinatal Exposure to Antiretroviral Drugs: Current Challenges and New Approaches
Journal of Neuroimmune Pharmacology (2021)
-
Birth defects in a cohort of infants born to HIV-infected women in Spain, 2000-2009
BMC Infectious Diseases (2014)
-
Neurological and Psychiatric Adverse Effects of Antiretroviral Drugs
CNS Drugs (2014)
-
Encephalocele following a periconceptional exposure to efavirenz: a case report
Journal of Perinatology (2013)
-
Treatment of children with HIV infection
Current HIV/AIDS Reports (2007)