Abstract
Using whole exome sequencing, we confirmed a diagnosis of biotin-responsive basal ganglia disease (BBGD) accompanied by possible Kawasaki Disease. BBGD is an autosomal-recessive disease arising from a mutation of the SLC19A3 gene encoding the human thiamine transporter 2 protein, and usually manifests as subacute to acute encephalopathy. In this case, compound heterozygous mutations of SLC19A3, including a de novo mutation in one allele, was the cause of disease. Although a large number of genetic neural diseases have no efficient therapy, there are several treatable genetic diseases, including BBGD. However, to achieve better outcome and accurate diagnosis, therapeutic analysis and examination for disease confirmation should be done simultaneously. We encountered a case of possible Kawasaki disease, which had progressed to BBGD caused by an extremely rare genetic condition. Although the prevalence of BBGD is low, early recognition of this disease is important because effective improvement can be achieved by early biotin and thiamine supplementation.
Similar content being viewed by others
Introduction
Biotin-responsive basal ganglia disease (BBGD) is an autosomal-recessive disease, which presents as confusions, dysarthria, dysphagia and external ophthalmoplegia.1, 2, 3, 4 Brain images reveal symmetrically distributed bilateral lesions of the caudate nuclei, putamen and subcortical white matter. Children with this disease show marked clinical improvement when biotin and thiamine is administrated early in relation to the onset of symptoms. If left untreated, patients can experience severe intellectual disability or even death. It is now known that BBGD is due to mutation of the SLC19A3 gene that encodes human thiamine transporter 2.5 Nevertheless, the diagnosis of BBGD remains difficult because there are several diseases that present with basal ganglia lesions. Furthermore, the disease is often triggered by febrile illness, which makes it difficult to differentiate BBGD from other febrile diseases.
Here, we report a case of BBGD, in which we administered biotin and thiamine as therapeutic diagnosis and confirmed the diagnosis by whole exome sequencing (WES).
Materials and methods
Subject
Peripheral blood samples of the affected individual (II-2 in Figure 1a) and his parents (I-1 and I-2 in Figure 1a) were collected after obtaining written informed consent. Genomic DNA was extracted from peripheral blood leukocytes using Quick Gene-610L (Fujifilm, Tokyo, Japan) according to the manufacturer’s instructions. The biological parent–child relationship was confirmed by haplotype analysis (Supplementary Method). Total RNA from lymphoblastoid cell lines was obtained by the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA). Subsequently, the first strand complementary DNA was synthesized with SuperScript III First-Strand Synthesis System for the reverse transcription-polymerase chain reaction kit (Invitrogen, Carlsbad, CA, USA). The Institutional Review Board of the Yokohama City University School of Medicine approved this study.
Mutation analysis of SLC19A3. (a) Pedigree of the family with a SLC19A3 mutation. Square and circle denotes male and female, respectively. White and black symbols indicate unaffected and affected individuals, respectively. (b) Electropherograms of the SLC19A3 mutation. (c) The functional domain of human SLC19A3. The substitution of p.S155L is located within the transmembrane domain of hTHTR2. (d) The evolutionary conservation of the S155 and A399 in SLC19A3. A full color version of this figure is available at the Journal of Human Genetics journal online.
Whole exome sequencing
WES was performed in the affected individual (II-2 in Figure 1a) as previously described.6 In brief, genomic DNA (3 μg for each sample) extracted from peripheral blood was sheared to 200-bp using a Covaris S2 system (Covaris, Woburn, MA, USA). The genome partitioning was performed using the SureSelect Human All Exon Kit v5 (Agilent Technologies, Santa Clara, CA, USA). The prepared libraries were sequenced on a Hiseq2000 (Illumina, San Diego, CA, USA) with 101-bp paired-end reads with 7-bp index reads. Both reads were aligned to the human reference genome hg19 by Novoalign 3.00 (http://www.novocraft.com). The aligned reads were processed by Picard to remove PCR duplicates (http://picard.sourceforge.net). The variants were called using the Genome Analysis Toolkit 1.6–5 (GATK; http://www.broadinstitute.org/gatk) with the GATK Best Practice Variant Detection v3 recommendations (http://www.broadinstitute.org/gatk/guide/topic?name=best-practices) and annotated by ANNOVAR (8 March 2012) (http://www.openbioinformatics.org/annovar). Using these criteria, only variants located in the coding region and the adjacent 2 bp were extracted, and common variants registered in dbSNP build 137 (Minor allele frequency ⩾0.01) (http://genome.ucsc.edu/cgi-bin/hgTrackUi?hgsid=316787363&g=snp137Common&hgTracksConfigPage=configure) were excluded.
Sanger sequencing
Candidate variants were validated in family members by Sanger sequencing. PCR products amplified with genomic DNA as a template were sequenced on an ABI3500xl autosequencer (Applied Biosystems, Foster City, CA, USA) and analyzed using Sequencher 5.0 (Gene Codes Corporation, Ann Arbor, MI, USA).
RNA analysis
Cloning analysis was used to confirm the parental origin of the patient’s mutant allele. First, reverse transcription-PCR was performed with ExTaq DNA polymerase (Takara Bio Inc., Otsu, Japan) on the complementary DNA of the patient. Primers were designed to contain both of the identified mutations in SLC19A3 (forward primer sequences; 5′-TGTTGTTTGGCCAAGGAGT-3′ and reverse primer sequences; 5′-TCTGGGATTTGGTTGAGTAGGT-3′). Second, the reverse transcription-PCR product was cloned into PCR 2.1-TOPO vector with the TOPO TA-Cloning kit (Invitrogen). Finally, 20 colonies were picked and the DNA in each clone was directly amplified with M13 primers and sequenced.
Results
Clinical course
A 6-month-old male infant visited our center because of a 3-day history of fever. The patient was the second son of healthy parents, and the older brother at the time of admission was 5-year-old. The father of the patient was in his 40 s and the mother was in her 20 s. After a monitored and uneventful pregnancy, the boy was delivered at 38 gestational weeks by vaginal delivery and weighed 3545 g. The boy had a health check-up when he was 3 months old and everything was determined to be normal. At the age of 5 months, the boy began to bend backward when his mother carried him. When he came to our center at the age of 6 months, he weighed 8.0 kg, appeared irritable and his body temperature was 38.5 °C with cold peripheral limbs. There were no abnormal data except for slightly elevated serum and cerebrospinal fluid lactate levels. (Supplementary Table 1) The results of serum amino-acid analysis and urine organic acid analysis were normal.
After admission, the patient started to punch his bed with his left hand, and right arm rigidity was observed. Moreover, deep tendon reflex was increased, and the patient lost consciousness. A head computed tomography scan revealed bilateral low signal regions of subcortical white matter, thalami and basal ganglia (Figure 2a). The patient was then admitted to the pediatric intensive care unit and closely monitored. The next day, the patient’s high fever continued and symptoms of Kawasaki Disease appeared (redness in eyes, rash on the body surface, swollen palms of the hand). At the same time, his whole-body rigidity increased, and he fell into respiratory failure. The patient was subsequently intubated and provided with respiratory care. Midazolam and fentanyl citrate were used for sedation and analgesia during respiratory care.
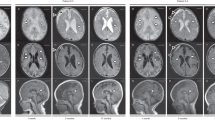
Head CT and MRI. (a–c) is taken at acute phase. (d–f) were taken 3 months later. (a) Axial head CT revealed the bilaterally low signal in the subcortical white matter, thalami and basal ganglia. (b) Axial head MRI. Diffusion weighted image (DWI). High signal was detected in the region consistent with the low signal region detected in the head CT. (c) Axial head MRI. Apparent diffusion coefficient (ADC) map. High signal was detected in the basal ganglia, whereas low signal was detected in the subcortical white matter (arrowhead). (d) Axial head MRI. Fluid-attenuated inversion recovery image. Mild atrophy was observed compared with the CT taken in acute phase. (e) Axial head MRI. Diffusion weighted image (DWI). High signal in basal ganglia and subcortical white matter in acute phase disappeared. (f) Axial head MRI. Apparent diffusion coefficient (ADC) map. Low signal in subcortical white matter in acute phase disappeared. The five brain images are sliced at approximately same level.
Considering the possibility of Kawasaki Disease, we started intravenous immunoglobulin therapy: 2 g kg−1 per day (two times) and flurbiprofen 5 mg kg−1 per day. Subsequently, the symptoms of Kawasaki Disease disappeared and the patient’s body temperature declined. Head magnetic resonance imaging taken 24 h after hospitalization showed bilateral and symmetric cortico-subcortical lesions involving the bilateral putamen and medial thalami (Figures 2b and c). Moreover, some brain regions showed low apparent diffusion coefficient values, suggesting an acute process. Other brain regions had high apparent diffusion coefficient values due to subacute or chronic processes. With the suspicion of mitochondrial encephalopathy, a combination of thiamine 100 mg per day, biotin 20 mg per day, ubidecarenone (CoQ10), tocopherol acetate (Juvela:vitamin E), levocarnitine chloride (L-Cartin), riboflavin butyrate (Hibon:vitamin B2), ascorbic acid (Hicee:vitamin C) and L-arginine hydrochloride (Argi-U) was started.
As the fever alleviated, the patient’s muscle rigidity decreased and his respiratory condition became stable. After his respiratory condition recovered completely, respiratory care was stopped on day 9 after admission. Furthermore, phenobarbital was administered to prevent convulsion after terminating the use of midazolam and fentanyl citrate. When the patient was discharged from the hospital on day 24 after admission, he was able to smile and could recognize his parents. His deep tendon reflux was still slightly increased. After he was discharged, he was continued on a combination of thiamine (100 mg per day), biotin (20 mg per day) and the other drugs indicated above. A follow-up magnetic resonance imaging at the age of 10 months revealed a mild atrophied region that was active in the acute phase (Figures 2d–f). The boy is now 1 year and 1 month old, and is capable of rolling over and sitting down for a while. Although the boy still cannot speak, his development is catching up to other children his age.
Mutation detection
As a consequence of WES, 94.4% of the coding sequence was covered by at least 20 reads in the affected individual. By filtering the variant for 41 known disease genes (Supplementary Table 2) for BBGD or Leigh syndrome, four variants of three genes were identified (Table 1). Among these variants, a synonymous variant in PDP1 (c.306C>T) and one splicing variant in NDUFAF6 (c.420+2->A), which was observed in 566 individuals of our in-house Japanese exome database (n=575), were excluded from the candidate variants. The remaining two heterozygous missense mutations were found in SLC19A3 (NM_025243.3): c.464C>T, p.Ser155Leu and c.1196A>T, p.Asn399Ile. SLC19A3 mutations are known to cause autosomal-recessive BBGD. These variants were not registered in our in-house exome database, 1000 Genomes database, or NHLBI Exome Sequencing Project (ESP6500).
A Sanger sequence of the SLC19A3 mutation showed that one variant (c.464C>T) occurred de novo in the patient, and that the other variant (c.1196A>T) was inherited from his mother (Figure 1b). Sequencing of complementary DNA from the patient’s lymphoblastoid cell line revealed that the de novo variants located on the allele differed from the maternal allele with c.1196A>T (Supplementary Figure 1). Therefore, the c.464C>T mutation was located on the paternal alleles. In conclusion, the patient had compound heterozygous SLC19A3 mutations. The de novo variant (p.Ser155Leu) in SLC19A3 was predicted to be pathogenic by three different programs (SIFT, Polyphen2 and MutationTaster) (Table 1).7, 8, 9 Serine155 is conserved from frogs to humans, and is located in the transmembrane domain (Figure 1c).10 The other variant (p.Asn399Ile), which was inherited from the patient’s mother, was predicted to be polymorphic (Table 1). However, we thought that this variant was pathogenic because the low prediction scores of pathogenicity might have been due to low conservation. The serine residue that is conserved from zebrafish to mice is replaced by asparagine in humans (Figure 1d). However, both amino residues are hydrophilic and the Grantham score between serine and asparagine is 46. Interestingly, the hydrophilic asparagine was changed to hydrophobic isoleucine in the patient, and the Grantham score between asparagine and isoleucine was 149 (Supplementary Table 3). Therefore, pathogenicity was very likely.
Discussion
Using WES, we confirmed a diagnosis of rare BBGD triggered by possible Kawasaki Disease. BBGD is an autosomal-recessive disorder that was first reported by Ozand et al.1 in 1998. In their report, biotin was markedly effective in half of the patients who had an initial diagnosis of mitochondrial disease. After Zeng et al.5 reported that BBGD is due to a mutation of SLC19A3, thiamine was added to the treatment indication, which led to better outcomes. SLC19A3 mutations are also found in Wernicke-like encephalopathy, Leigh encephalopathy, infantile spasms and infantile encephalopathy.2, 11, 12 In Japan, Yamada et al.13 reported four patients from one pedigree who had the SLC19A3 mutation.
In this case, we concluded that compound hetereozygous mutations of SLC19A3 were the cause of the disease. We demonstrated that one of the mutations arose de novo, by cloning PCR products containing paternal SNP. Furthermore, bioinformatic analysis predicted that amino-acid replacement was the cause of this disease. Although, it has been reported that the rate of de novo mutations correlate with a father’s age, de novo mutations triggering autosomal-recessive diseases in children, which is a unique feature of this case, is rare.14 From a genetic counseling point of view, we would inform the family that the possibility that any subsequent child would present BBGD by similar mutations would be extremely low, as one of the mutations in this case arose de novo.
Although a large number of genetic nervous diseases have no efficient therapy, there are several treatable genetic diseases that are related to the soluble vitamin (biotin, thiamine, folic acid) gene, including BBGD.15, 16, 17 To achieve better outcomes in these diseases, both therapeutic diagnosis and confirmation studies should be conducted simultaneously. In this case, considering the possibility of mitochondrial encephalopathy, we first started biotin and thiamine therapy and then carried out WES analysis for diagnosis. Regarding the BBGD treatment, Ozand et al.1 reported the dose of biotin has to be 5–10 mg kg−1 per day, whereas subsequent studies used lower doses of biotin (2–3 mg kg−1 per day) in addition to thiamine (100–300 mg per day) with the same efficacy.4 In this case, it was difficult to determine whether the therapy improved the patient’s outcome or whether we just observed the disease’s natural course. However, considering that no follow-up attacks or aggravation in magnetic resonance imaging were seen in this patient, there should be no conflict that biotin and thiamine was effective in disease treatment. Importantly, we concluded that diagnosis of BBGD strongly indicated continuation of biotin-thiamine therapy in this patient.
In the current case, along with BBGD, we considered of mitochondrial diseases as differential diagnosis. Therefore, we carried out whole exome sequence. By filtering the variant for 41 known disease genes for BBGD or Leigh syndrome, we were able to diagnose BBGD. Nevertheless, it is expensive and time consuming to run these tests in every similar case. In the prospective view, it would be useful to develop a gene analysis panel tool that could be used for treatable diseases, including BBGD, that are related to the soluble vitamin gene.
Although BBGD is due to a mutation of SLC19A3, the gene encoding human thiamine transporter 2, it shows a biotin-responsive clinical course. This is one of the biggest questions still remaining because Subramanian et al.18 showed that biotin is not a substrate of human thiamine transporter 2. When mitochondria become deficient of thiamin, the reaction of pyruvate dehydrogenase toward the tricarboxylic acid cycle via Acetyl CoA is reduced (Supplementary Figure 2). One hypothesis is that, in this condition, supplying a high dose of biotin enables pyruvate to bypass tricarboxylic acid cycle by using pyruvate carboxylase, which produces oxaloacetate. Oxaloacetate is supplied as a substrate in the tricarboxylic acid cycle instead of acetyl CoA and as a result, alleviates the symptoms of BBGD. Further research is required to clarify the mechanism of biotin efficacy.
Using next-generation sequence analysis, we confirmed a diagnosis of BBGD accompanied by possible Kawasaki Disease. In the process of diagnosis, we could detect compound heterozygous mutations of SLC19A3 including a de novo mutation in one allele. Although the prevalence is low, recognition of BBGD is important because effective improvement can be achieved by early biotin and thiamine supplementation.
References
Ozand, P. T., Gascon, G. G., Essa, M. A., Joshi, S., Jishi, E. A. & Bakheet, S. et al. Biotin-responsive basal ganglia disease: a novel entity. Brain 121, 1267–1279 (1998).
Perez-Duenas, B., Seeeano, M., Rebollo, M., Muchart, J., Gargallo, E. & Dupuits, C. et al. Reversible lactic acidosis in a newborn with thiamine transporter-2 deficiency. Pediatrics 131, e1670–e1675 (2013).
Alfadhel, M., Almuntashri, M., Jadah, R. H., Bashiri, F. A., Rifai, M. T. A. & Shalaan, H. A. et al. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J. Rare Dis. 8, 83 (2013).
Tabarki, B., Al-Shafi, S., Al-Shahwan, S., Azmat, Z., Al-Hashem, A. & Al-Adwani, N. et al. Biotin-responsive basal ganglia disease revisited. Neurology 80, 261–267 (2013).
Zeng, W. Q., Al-Yamani, E., Acierno, J. S., Slaugenhaupt, S., Gillis, T. & MacDonald, M. E. et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am. J. Hum. Genet. 77, 16–26 (2005).
Imagawa, E., Osaka, H., Yamashita, A., Shiina, M., Takahashi, E. & Sugie, H. et al. A hemizygous GYG2 mutation and Leigh syndrome: a possible link? Hum. Genet. 133, 225–234 (2014).
Ng, P. C. & Heinkoff, S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. Vol. 31, 3812–3814 (2003).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A. & Bork, P. et al. A method and server for predicting damaging missense mutations. Nat. Methods Vol. 7, 248–249 (2010).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods Vol. 7, 575–576 (2010).
Subramanian, V. S., Marchant, J. S. & Said, H. M. Targeting and trafficking of the human thiamin transporter-2 in epithelial cells. J. Biol. Chem. 281, 5233–5245 (2006).
Kono, S., Miyajima, H., Yoshida, K., Togawa, A., Shirakawa, K. & Suzuki, H. Mutations in a thiamine-transporter gene and Wernicke’s-like encephalopathy. N. Engl. J. Med. 360 (17), 1792–1794 (2009).
Gerards, M., Kamps, R., Oevelen, J., Boesten, I., Jongen, E. & Koning, B. et al. Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain 136, 882–890 (2013).
Yamada, K., Miura, K., Hara, K., Suzuki, M., Nakanishi, K. & Kumagai, T. et al. A wide spectrum of clinical and brain MRI findings in patient with SLC19A3 mutations. BMC Med. Genet. 11, 171 (2010).
Kong, A., Frigge, M., Masson, G., Soren, B., Sulem, P. & Giski, M. et al. Rate of de novo mutations and the importance of father’s age to disease. Nature 488, 471–475 (2012).
Wolf, B. & Feldman, G. L. The biotin-dependent carboxylase deficiencies. Am. J. Hum. Genet. 34, 699–716 (1982).
Scriver, C. R., MacKenize, S., Clow, C. L. & Delvin, E. Thiamine-responsive maple-syrup-urine disease. Lancet 297, 310–312 (1971).
Corbeel, L., Van den Berghe, G., Jaeken, J., VanTornout, J. & Eeckels, R. Congenital folate malabsorption. Eur. J. Pediatr. 143, 284–290 (1985).
Subramanian, V. S., Marchant, J. S. & Said, H. M. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am. J. Physiol. Cell. Physiol. 291, C851–C859 (2006).
Acknowledgements
We appreciate the support of Satoko Kumada at the Tokyo Metropolitan Neurological Hospital for helping us to make an accurate diagnosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Kohrogi, K., Imagawa, E., Muto, Y. et al. Biotin-responsive basal ganglia disease: a case diagnosed by whole exome sequencing. J Hum Genet 60, 381–385 (2015). https://doi.org/10.1038/jhg.2015.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2015.35
This article is cited by
-
A Japanese patient with neonatal biotin-responsive basal ganglia disease
Human Genome Variation (2022)