Abstract
Costello syndrome is an autosomal dominant disorder comprising growth deficiency, mental retardation, curly hair, coarse facial features, nasal papillomata, low-set ears with large lobes, cardiac anomalies, redundant skin in palms and soles with prominent creases, dark skin, and propensity to certain solid tumors. HRAS mutations have been implicated in approximately 85% of the affected cases. The clinical overlap among Costello, Noonan, and cardiofaciocutaneous syndromes is now better understood given their common molecular background, such that all these syndromes constitute a class of disorders caused by deregulated RAS-MAPK signaling. We report on a novel KRAS gene mutation in a patient presenting the clinical features typical of Costello syndrome and the additional findings seen in Noonan syndrome. This description emphasizes that a subset of patients with Costello syndrome could harbor mutations in other genes involved in the RAS-MAPK signaling.
Similar content being viewed by others
Introduction
Costello syndrome (CS; OMIM 218040) is characterized by postnatal growth deficiency, mental retardation, curly hair, coarse facial features, nasal papillomata, low-set ears with large lobes, cardiac anomalies, redundant skin in palms and soles with prominent creases, dark skin, and propensity to certain solid tumors (Costello 1996). The gene responsible for the disorder was recently identified as the HRAS gene, an oncogene whose codified proteins are involved in the mitogen-activated protein kinase (MAPK) pathway (Aoki et al. 2005).
In different cohorts of CS patients, mutations in the HRAS gene were identified in 82.5–92%, indicating that this gene is the main one responsible for this phenotype (Aoki et al. 2005; Gripp et al. 2006).
The clinical features of CS overlap with those found in Noonan syndrome (NS). For several decades there has been a debate as to whether CS and other disorders, such as LEOPARD, cardiofaciocutaneous (CFC) and Noonan-like/multiple giant cell lesion syndromes, were distinct entities or part of the spectrum of NS (Costello 1996; Neri et al. 1991; Bertola et al. 2001).
The recent identification of genes responsible for Noonan syndrome and the other Noonan-like syndromes has shed light on these overlapping phenotypes. It is now known that dysregulation of the RAS-MAPK signaling is the pivotal molecular mechanism in the etiology of these related disorders. In NS, the identified genes of this signaling pathway are PTPN11 (Tartaglia et al. 2001), KRAS (Schubbert et al. 2006), and SOS1 (Tartaglia et al. 2006; Roberts et al. 2006).
A detailed description of patients affected by these disorders and their molecular defects is important for the development of a more specific genotype–phenotype correlation. This has real implications in the follow-up of the patients, as it is well known that CS and, therefore, mutations in HRAS gene, give rise to an increased risk of solid tumors. This tendency is not observed in individuals with mutations in other genes responsible for CFC syndrome, such as BRAF, MEK1, and MEK2 (Niihori et al. 2006; Rodriguez-Viciana et al. 2006).
Here, we report on a proband with clinical features compatible with Costello syndrome presenting a new mutation in the KRAS gene.
Case report
The patient was part of a previously published study of PTPN11 gene mutations in 74 NS and Noonan-like syndrome cases (Bertola et al. 2006).
The proband, a 20-year-old woman, is the fifth child of nonconsanguineous parents. She was born at term, by cesarean section, after a pregnancy with polyhydramnios detected in the last trimester. Birth weight was 2,100 g and length 47 cm. A cardiac murmur was audible soon after birth, and a diagnosis of hypertrophic cardiomyopathy with dysplastic pulmonary and mitral valves was disclosed.
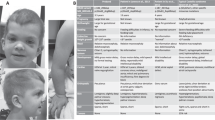
She evolved with severe developmental delay. Her heart condition remained stable with the use of β-blockers, showing no signs of outflow obstruction. At the age of 15 years, a lymphedema started in her right leg, limited to below the knee with a rapid increase in volume and subsequent involvement of the contralateral leg. The use of below-the-knee elastic stockings led to no improvement. Three years later, nasal papillomata developed in her right nostril (Fig. 1).
She was first seen in our clinic at the age of 8 years, with a weight of 15,500 g (<2.5th centile), height of 107.5 cm (<2.5th centile) and occipitofrontal circumference of 52.2 cm (50th centile). She presented with extremely curly hair, coarse facial features with ocular hypertelorism, down-slanting palpebral fissures, palpebral ptosis, proptosis, prominent lips, fleshy earlobes, webbed neck, pectus excavatum, hyperextensible joints, loose skin in hands and feet, deep creases in the palms and soles and dark skin (Fig. 1). Additional testing revealed a normal abdominal ultrasound, spine X-ray, audiogram, G-banded karyotype, and hematological evaluation. Ophthalmologic evaluation showed prominent corneal nerves, and a cranial CT scan disclosed ventriculomegaly.
The initial diagnosis was Noonan syndrome, but the presence of relative macrocephaly, coarse facial features, loose skin in the hands and feet with deep creases, dark skin, and especially, the development of nasal papillomata led to the diagnosis of Costello syndrome.
The family history is remarkable for the fact that the patient has only one living brother, product of her mother’s second pregnancy; his physical exam and echocardiogram were normal. The first pregnancy was a stillbirth. The third child died in the neonatal period presenting heart defect (VSD, ASD and PDA) and no facial dysmorphisms according to the mother. No photographs had been taken. Interestingly, another deceased brother showed facial features very similar to the patient. He died due to a severe hypertrophic cardiomyopathy with outflow obstruction. The mother has mitral valve prolapse, but no other signs suggestive of Costello syndrome. The father is deceased and had never been available for a genetic evaluation. Photographs failed to show a typical phenotype of Noonan or Costello syndromes, and there was no history of cardiac problems.
Methods
Venous blood was drawn for genomic DNA extraction after informed consent was obtained from the proband’s mother. DNA extraction from peripheral leukocytes was performed using a salting-out protocol.
Because the initial diagnosis was NS, sequencing of PTPN11 gene was performed. Mutational analysis of the PTPN11 gene was performed using denaturing high-performance liquid chromatography (DHPLC; Transgenomic, Cheshire, UK) for screening exons potentially carrying a mutation. Gradients and column temperatures were used as recommended by the Wavemaker 4.1 software. Amplicons with an aberrant elution profile were subsequently sequenced. Bidirectional direct sequencing of purified PCR products was carried out in suspect exons using the ABI Terminator Sequencing Kit, an ABI 377 Sequencer, and primers specific for each exon of the PTPN11 gene.
The HRAS gene was also analyzed. Bidirectional direct sequencing of purified PCR products for the coding exons of the HRAS gene (exons 2, 3, 4, and 5) was carried out using the ABI Terminator Sequencing Kit, an ABI 377 Sequencer, and primers specific for each coding exon of the HRAS gene.
After the identification of KRAS as a gene potentially involved in NS and/or NS-like phenotypes, we proceeded to sequence the KRAS gene (exons 2, 3, 4, 5, and 6) using the same method described above for HRAS gene.
Identified genetic alterations were confirmed initially through repeated bidirectional sequencing and secondly through the sequencing of cloned alleles in pCRScript cloning vectors.
Results
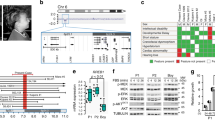
No mutations were found in the coding regions of the PTPN11 and HRAS genes. An A → G transversion at nucleotide position 194 of KRAS cDNA, predicting a K5E substitution, was found in exon 2 of the KRAS gene in the proband. Her mother and brother (the only family members also available for genetic testing) did not harbor this mutation (Fig. 2a).
This KRAS gene alteration was not found in 100 controls also subjected to KRAS exon 2 sequencing. In addition, this particular genetic alteration was confirmed by the cloning and sequencing of each allele of the proband in cloning vectors (Fig. 2b).
Discussion
The definitive diagnosis of a specific syndrome is not always straightforward. The variable expressivity in autosomal dominant disorders, allied with the fact that apparently different disorders overlap in their clinical findings, makes establishing a diagnosis difficult.
This occurred in our case, with an initial diagnosis of NS. However, the presence of relative macrocephaly, coarse facial features, thick lobes, nasal papillomata, prominent lips, hyperextensible joints, loose skin in hands and feet, deep creases in palms and soles, and dark skin are all typical features of CS. Nevertheless, it should also be noted that our patient presented lymphedema and prominent corneal nerves, characteristics described in NS with a frequency of 20% (Mendez and Opitz 1985) and 40% (Bertola et al. 2006) respectively, but not in CS.
In the family history of our case, we observed unquestionable recurrence of the phenotype in a deceased brother. The mother did not harbor a KRAS gene mutation, but, unfortunately, the father was not available for the molecular test. Although photographs of the father did not show typical findings of the disease, we cannot rule out the diagnosis with certainty due to variable expressivity of the phenotype. On the other hand, there is a possibility of gonadal mosaicism. This mechanism in CS was initially described by Lurie (1994), who characterized this syndrome as an autosomal dominant disorder. Initially, CS was considered an autosomal recessive disorder, due to the fact that in some families there was a history of consanguinity and also two instances of sibship recurrence.
The underlying molecular defect can help split or lump disorders that are clinically related in a more accurate way, and great efforts have been expended to this end. Gelb and Tartaglia (2006), in a recent review of the new knowledge involving NS and its related syndromes, concluded that all of these syndromes constitute a class of disorders caused by deregulated RAS-MAPK signaling.
It is unquestionable that HRAS is the main gene involved in CS, proven by the exceptionally high prevalence of mutations found in the affected patients (Aoki et al. 2005; Gripp et al. 2006; Kerr et al. 2006). The codons 12 and 13 are repeatedly involved as the sites of mutations in CS. The main gene alteration found is G12S, present in 85% of the cases studied (Kerr et al. 2006). Nevertheless, a small portion of these clinically diagnosed CS patients do not harbor an HRAS mutation. It is conceivably possible that these cases were atypical (i.e., patients that do not harbor an HRAS mutation had some clinical features of CFC syndrome) as indicated by Gripp et al. (2006).
The main clinical characteristics found in CS patients with HRAS mutations are developmental delay/mental retardation, failure to thrive, characteristic facies, and classic skin anomaly, observed in all cases (Aoki et al. 2005; Gripp et al. 2006; Estep et al. 2006; van Steensel et al. 2006; Zampino et al. 2006). Other frequent findings are short stature (90%), curly/sparse hair (94%), hand anomaly (89%), polyhydramnios (88%), and cardiac abnormality (70%), mainly hypertrophic cardiomyopathy (Table 1).
Three patients with a Costello phenotype and mutations in KRAS gene were described. Our case is the fourth.
Carta et al. (2006) reported a patient with a severe Noonan syndrome phenotype with overlapping features of sCS and CFC syndrome. The proband, harboring a V152G KRAS gene mutation, presented some characteristics of CS, such as polyhydramnios, failure to thrive, relative macrocephaly, and suggestive facial features, but lacked the skin and cardiac anomalies. Further evidence that CFC syndrome and CS share several clinical findings was reported in the study of Narumi et al. (2007). In 35 CFC patients showing mutations in BRAF, KRAS and MEK-1, and MEK-2, approximately 40% presented wrinkled palms and soles, hyperpigmentation, and joint hyperextension, characteristics frequently seen in CS but not in CFC syndrome.
Zenker et al. (2006) described two infants with typical findings of CS presenting different mutations in the KRAS gene. They both presented failure to thrive, developmental delay, characteristic facies, and skin and cardiac anomalies. Interestingly, one of them harbored a mutation in the same codon (5) involved in our case. It would be very interesting to observe whether this patient develops any characteristic of NS in the future, similar to our case. Although the germline KRAS mutations described in NS and CFC syndrome hardly overlap with the somatic mutations found in cancer, the K5N has been previously described as the probable causative mutation in gastric cancer. Therefore, it is possible that this mutation predisposes patients to an increased tumor risk, as is common in CS.
The K5E mutation found in our case was not described as a somatic event in cancer (Sanger Institute Catalogue of Somatic Mutations in Cancer, 2005), but a close surveillance should be performed in this patient.
Also, Rauen (2006) studied three patients—who met criteria for the diagnosis of CS and were HRAS-mutation negative—for mutations in other genes involved in the RAS-MAPK pathway: BRAF, MEK1, MEK2, and KRAS. Two of them showed gene alterations in the BRAF gene, usually involved in cases of CFC syndrome. The mutations were, however, located in different exons from the ones found previously in CFC syndrome patients.
As in the case reported by Rauen (2006), the mutation in KRAS gene found in our patient is novel, not described previously in individuals with either NS or CFC syndrome. Therefore, the involvement of BRAF or, in our case, the KRAS gene in patients with CS suggests that these genes could also be responsible for a Costello phenotype, possibly with clinical findings less typical of CS, or, as in our case, with additional findings seen in NS.
The clinical findings described in CS patients with KRAS gene mutations published so far are detailed in Table 2. Developmental delay/mental retardation, failure to thrive, short stature, relative macrocephaly, characteristic facies, and curly/sparse hair were characteristics present in all probands, very similar to the ones with HRAS gene mutations.
Based on these data, it is still not possible to draw definite conclusions about the genotype–phenotype correlation. Certainly, the typical cases of CS have an enormous probability of harboring a HRAS gene mutation, with an increased risk for the development of solid tumors.
Kerr et al. (2006) suggested a potential correlation between mutation type and malignancy in CS. They showed that the most common mutation (G12S) had a frequency of tumor development of 7%, but the mutation G12A a frequency of 57%.
Although none of the patients harboring a KRAS mutation developed cancer, including our 20-year-old patient, it is still premature to affirm that mutations in this gene do not predispose to malignancy. The very small size of this cohort, the fact that two of the patients were infants, and the possibility that K5N somatic mutation is responsible for gastric cancer do not allow a definite conclusion to be drawn.
Further reports of these syndromes, with their molecular studies, should help to establish a better delineation of the disorders and allow a more accurate genotype–phenotype correlation to be defined with beneficial effects in genetic counseling for the affected individuals and families.
References
Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y et al (2005) Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet 37:1038–1040
Bertola DR, Kim CA, Pereira AC, Mota GF, Krieger JE, Vieira IC et al (2001) Are Noonan syndrome and Noonan-like/multiple giant cell lesion syndrome distinct entities? Am J Med Genet 98:230–234
Bertola DR, Pereira AC, Albano LM, De Oliveira PS, Kim CA, Krieger JE (2006) PTPN11 gene analysis in 74 Brazilian patients with Noonan syndrome or Noonan-like phenotype. Genet Test 10:186–191
Carta C, Pantaleoni F, Bocchinfuso G, Stella L, Vasta I, Sarkozy A et al (2006) Germline missense mutations affecting KRAS isoform B are associated with a severe Noonan syndrome phenotype. Am J Hum Genet 79:129–135
Costello JM (1996) Costello syndrome: update on the original cases and commentary. Am J Med Genet 62:199–201
Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA (2006) HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A 140:8–16
Gelb BD, Tartaglia M (2006) Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet 15 (Spec No 2):R220–R226
Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CI Jr, Doyle D et al (2006) HRAS mutation analysis in Costello syndrome: genotype and phenotype correlation. Am J Med Genet A 140:1–7
Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I et al (2006) Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet 43:401–405
Lurie IW (1994) Genetics in Costello syndrome. Am J Med Genet 52:358–359
Mendez HMM, Opitz JM (1985) Noonan syndrome: a review. Am J Med Genet 21:493–506
Narumi Y, Aoki Y, Niihori T, Neri G, Cave H, Verloes A et al (2007) Molecular and clinical characterization of cardio-facio-cutaneous (CFC) syndrome: overlapping clinical manifestations with Costello syndrome. Am J Med Genet A 143(8):799–807
Neri G, Zollino M, Reynolds JF (1991) The Noonan-CFC controversy. Am J Med Genet 39:367–370
Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A et al (2006) Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet 38:294–296
Rauen KA (2006) Distinguishing Costello versus cardio-facio-cutaneous syndrome: BRAF mutations in patients with a Costello phenotype. Am J Med Genet A 140:1681–1683
Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA et al (2006) Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet 39(1):70–74
Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS et al (2006) Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science 311:1287–1290
Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G et al (2006) Germline KRAS mutations cause Noonan syndrome. Nat Genet 38:331–336
Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H et al (2001) Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet 29:465–468
Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A et al (2006) Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet 39(1):75–79
van Steensel MA, Vreeburg M, Peels C, van Ravenswaaij-Arts CM, Bijlsma E, Schrander-Stumpel CT et al (2006) Recurring HRAS mutation G12S in Dutch patients with Costello syndrome. Exp Dermatol 15:731–734
Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C et al (2006) Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat 28(3):265–272
Zenker M, Lehmann K, Schulz AL, Barth H, Hansmann D, Koenig R et al (2006) Expansion of the genotypic and phenotypic spectrum in patients with KRAS germline mutations. J Med Genet 44(2):131–135
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertola, D.R., Pereira, A.C., Brasil, A.S. et al. Further evidence of genetic heterogeneity in Costello syndrome: involvement of the KRAS gene. J Hum Genet 52, 521–526 (2007). https://doi.org/10.1007/s10038-007-0146-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-007-0146-1
Keywords
This article is cited by
-
The impact of RASopathy-associated mutations on CNS development in mice and humans
Molecular Brain (2019)
-
Concurrent somatic KRAS mutation and germline 10q22.3-q23.2 deletion in a patient with juvenile myelomonocytic leukemia, developmental delay, and multiple malformations: a case report
BMC Medical Genomics (2018)
-
Noonan syndrome caused by germline KRAS mutation in Taiwan: report of two patients and a review of the literature
European Journal of Pediatrics (2009)