Abstract
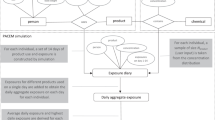
Exposure assessment is a key step in determining risks to chemicals in consumer goods, including personal care products (PCPs). Exposure models can be used to estimate exposures to chemicals in the absence of biomonitoring data and as tools in chemical risk prioritization and screening. We apply a PCP exposure model based on the product intake fraction (PiF), which is defined as the fraction of chemical in a product that is taken in by the exposed population, to estimate chemical intake based on physicochemical properties and PCP usage characteristics. The PiF can be used to estimate route and pathway-specific exposures during both the use and disposal stages of a product. As a case study, we stochastically quantified population level exposures to parabens in PCPs, and compared estimates with biomarker values. We estimated exposure based on the usage of PCPs in the female US population, taking into account population variability, product usage characteristics, paraben occurrence in PCPs and the PiF. Intakes were converted to urine levels and compared with National Health and Nutrition Examination Survey (NHANES) biomonitoring data. Results suggest that for parabens, chemical exposure during product use is substantially larger than environmentally mediated exposure after product disposal. Modeled urine concentrations reflect well the NHANES variation of three orders of magnitude across parabens for the 50th, 75th, 90th, and 95th percentiles and were generally in good agreement with measurements, when taking uncertainty into account. This study presents an approach to estimate multi-pathway exposure to chemicals in PCPs and can be used as a tool within exposure-based screening of chemicals as well in higher tier exposure estimates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cowan-Ellsberry CE, Robison SH . Refining aggregate exposure: example using parabens. Regul Toxicol Pharmacol 2009; 55: 321–329.
Gosens I, Delmaar CJE, Ter Burg W, de Heer C, Schuur AG . Aggregate exposure approaches for parabens in personal care products: a case assessment for children between 0 and 3 years old. J Expo Sci Environ Epidemiol 2014; 24: 208–214.
Wambaugh JF, Setzer RW, Reif DM, Gangwal S, Mitchell-Blackwood J, Arnot JA et al. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ Sci Technol 2013; 47: 8479–8488.
Isaacs KK, Glen WG, Egeghy P, Goldsmith M-R, Smith L, Vallero D et al. SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ Sci Technol 2014; 48: 12750–12759.
Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K et al. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 2012; 125: 157–174.
Shin H-M, Ernstoff A, Arnot JA, Wetmore BA, Csiszar SA, Fantke P et al. Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ Sci Technol 2015; 49: 6760–6771.
Shin H-M, McKone TE, Bennett DH . Intake fraction for the indoor environment: a tool for prioritizing indoor chemical sources. Environ Sci Technol 2012; 46: 10063–10072.
Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R . Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol 24: 459–466.
Parlett LE, Calafat AM, Swan SH . Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 2013; 23: 197–206.
Wormuth M, Demou E, Scheringer M, Hungerbühler K . Assessments of direct human exposure—the approach of EU risk assessments compared to scenario based risk assessment. Risk Anal 2007; 27: 979–990.
Jolliet O, Fantke P . Human toxicity. In: Hauschild MZ, Huijbregts MAJ (eds). Life Cycle Impact Assessment. Springer Press: Dordrecht. 2015, pp 75–96.
Guo Y, Kannan K . A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol 2013; 47: 14442–14449.
Ten Berge W . A simple dermal absorption model: derivation and application. Chemosphere 2009; 75: 1440–1445.
Wilschut A, ten Berge WF, Robinson PJ, McKone TE . Estimating skin permeation. The validation of five mathematical skin permeation models. Chemosphere 1995; 30: 1275–1296.
Delmaar C, Bokkers B, Ter Burg W, Schuur G . Validation of an aggregate exposure model for substances in consumer products: a case study of diethyl phthalate in personal care products. J Expo Sci Environ Epidemiol 2014; 25: 317–323.
Tibaldi R, ten Berge W, Drolet D . Dermal absorption of chemicals: estimation by IH SkinPerm. J Occup Environ Hyg 2014; 11: 19–31.
Jolliet O, Ernstoff AS, Csiszar SA, Fantke P . Defining product intake fraction to quantify and compare exposure to consumer products. Environ Sci Technol 2015; 49: 8924–8931.
Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M et al. Risk assessment in the 21st century: roadmap and matrix. Crit Rev Toxicol 2014; 44: 6–16.
Soni MG, Carabin IG, Burdock GA . Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005; 43: 985–1015.
Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM . Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect 2006; 114: 1843–1846.
CDC (Centers for Disease Control and Prevention). Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables. US Department of Health and Human Services, 2014. Available from http://www.cdc.gov/exposurereport/.
Calafat AM, Ye X, Wong L-Y, Bishop AM, Needham LL . Urinary concentrations of four parabens in the U.S. population: NHANES 2005-2006. Environ Health Perspect 2010; 118: 679–685.
Boberg J, Taxvig C, Christiansen S, Hass U . Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 2010; 30: 301–312.
Błędzka D, Gromadzińska J, Wąsowicz W . Parabens. From environmental studies to human health. Environ Int 2014; 67: 27–42.
Rosenbaum R, Huijbregts M, Henderson A, Margni M, McKone T, van de Meent D et al. USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: sensitivity to key chemical properties. Int J Life Cycle Assess 2011; 16: 710–727.
Goldsmith M-R, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame et al. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem Toxicol 2014; 65: 269–279.
Bremmer HJ, Prud’homme de Lodder L, van Engelen J . Cosmetics Fact Sheet. To Assess the Risks for the Consumer. Updated Version for ConsExpo 4. RIVM Report 320104001/2006. National Institute for Public Health and the Environment: Bilthoven, The Netherlands. 2006.
Caon T, Costa ACO, de Oliveira MAL, Micke GA, Simões CMO . Evaluation of the transdermal permeation of different paraben combinations through a pig ear skin model. Int J Pharm 2010; 391: 1–6.
Kitagawa S, Li H, Sato S . Skin permeation of parabens in excised guinea pig dorsal skin, its modification by penetration enhancers and their relationship with n-octanol/water partition coefficients. Chem Pharm Bull (Tokyo) 1997; 45: 1354–1357.
Nanayakkara GR, Bartlett A, Forbes B, Marriott C, Whitfield PJ, Brown MB . The effect of unsaturated fatty acids in benzyl alcohol on the percutaneous permeation of three model penetrants. Int J Pharm 2005; 301: 129–139.
Nicoli S, Zani F, Bilzi S, Bettini R, Santi P . Association of nicotinamide with parabens: effect on solubility, partition and transdermal permeation. Eur J Pharm Biopharm 2008; 69: 613–621.
Dal Pozzo A, Pastori N . Percutaneous absorption of parabens from cosmetic formulations. Int J Cosmet Sci 1996; 18: 57–66.
Sugibayashi K, Todo H, Oshizaka T, Owada Y . Mathematical model to predict skin concentration of drugs: toward utilization of silicone membrane to predict skin concentration of drugs as an animal testing alternative. Pharm Res 2010; 27: 134–142.
Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD et al. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 2005; 43: 279–291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W et al. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol 2006; 44: 2008–2018.
Loretz LJ, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC et al. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 2008; 46: 1516–1524.
Rastogi SC, Schouten A, de Kruijf N, Weijland JW . Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Dermatitis 1995; 32: 28–30.
Wang L, Liao C, Liu F, Wu Q, Guo Y, Moon H-B et al. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ Sci Technol 2012; 46: 11584–11593.
Liao C, Liu F, Kannan K . Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol 2013; 47: 3918–3925.
Angerer J, Aylward LL, Hays SM, Heinzow B, Wilhelm M . Human biomonitoring assessment values: approaches and data requirements. Int J Hyg Environ Health 2011; 214: 348–360.
Søeborg T, Frederiksen H, Andersson A-M . Considerations for estimating daily intake values of nonpersistent environmental endocrine disruptors based on urinary biomonitoring data. Reproduction 2014; 147: 455–463.
Kirman CR, Aylward LL, Wetmore BA, Thomas RS, Sochaski M, Ferguson SS et al. Quantitative property–property relationship for screening-level prediction of intrinsic clearance: a tool for exposure modeling for high-throughput toxicity screening data. Appl Vitr Toxicol 2015; 1: 140–146.
Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW et al. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol Sci 2015; 148: 121–136.
Weschler CJ, Nazaroff WW . Dermal uptake of organic vapors commonly found in indoor air. Environ Sci Technol 2014; 48: 1230–1237.
El Hussein S, Muret P, Berard M, Makki S, Humbert P . Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study). Exp Dermatol 2007; 16: 830–836.
Gouin T, van Egmond R, Sparham C, Hastie C, Chowdhury N . Simulated use and wash-off release of decamethylcyclopentasiloxane used in anti-perspirants. Chemosphere 2013; 93: 726–734.
Arnot JA, Brown TN, Wania F . Estimating screening-level organic chemical half-lives in humans. Environ Sci Technol 2014; 48: 723–730.
Biesterbos JWH, Dudzina T, Delmaar CJE, Bakker MI, Russel FGM, von Goetz N et al. Usage patterns of personal care products: important factors for exposure assessment. Food Chem Toxicol 2013; 55: 8–17.
Manová E, von Goetz N, Keller C, Siegrist M, Hungerbühler K . Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int J Environ Res Public Health 2013; 10: 2778–2798.
US EPA. Estimation Programs Interface Suite for Microsoft Windows, v 4.11. United States Environmental Protection Agency: Washington, DC, USA, 2012.
Acknowledgements
We thank Barbara Wetmore (Hamner Institutes for Health Sciences) for the clearance values and Jacqueline Biesterbos and Nel Roeleveld (Radboud University) for product usage data. Funding was provided by the University of Michigan Risk Science Center, the Dow Postdoctoral Fellowship in Sustainability to SAC and the Long Range Research Initiative of the American Chemistry Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website
Supplementary information
Rights and permissions
About this article
Cite this article
Csiszar, S., Ernstoff, A., Fantke, P. et al. Stochastic modeling of near-field exposure to parabens in personal care products. J Expo Sci Environ Epidemiol 27, 152–159 (2017). https://doi.org/10.1038/jes.2015.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2015.85
Keywords
This article is cited by
-
A modular mechanistic framework for estimating exposure to SVOCs: Next steps for modeling emission and partitioning of plasticizers and PFAS
Journal of Exposure Science & Environmental Epidemiology (2022)
-
Exposure modelling in Europe: how to pave the road for the future as part of the European Exposure Science Strategy 2020–2030
Journal of Exposure Science & Environmental Epidemiology (2022)
-
Personal care product use among diverse women in California: Taking Stock Study
Journal of Exposure Science & Environmental Epidemiology (2021)
-
Exposure and toxicity characterization of chemical emissions and chemicals in products: global recommendations and implementation in USEtox
The International Journal of Life Cycle Assessment (2021)
-
Exposure to selected preservatives in personal care products: case study comparison of exposure models and observational biomonitoring data
Journal of Exposure Science & Environmental Epidemiology (2020)