Abstract
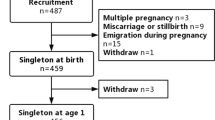
Breastfeeding has been associated with an advantage to infant neurobehavioral development, possibly in part due to essential nutrients in breast milk. However, breast milk may be contaminated by environmental neurotoxicants, such as methylmercury. In the Faroe Islands, where maternal consumption of pilot whale may cause transfer of marine toxicants into breast milk, a cohort of 1022 consecutive singleton births was generated during 1986–87. Methylmercury exposure was assessed from mercury concentrations in cord blood and in the hair of the child at age 12 months, and the duration of breastfeeding was recorded. At approximately 7 years of age, 917 (90%) of the children underwent detailed neurobehavioral examination. After adjustment for confounders, breastfeeding was associated with only marginally better neuropsychological performance on most tests. These associations were robust even after adjustment for cord-blood and hair mercury concentration at age 1 year. Thus, in this cohort of children with a relatively high prenatal toxicant exposure and potential exposure to neurotoxicants through breast milk, breastfeeding was associated with less benefits on neurobehavioral development than previously published studies though not associated with a deficit in neuropsychological performance at age 7. Although the advantage may be less, Faroese women can still safely breastfeed their children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Amin-Zaki L., Elhassani S.B., Majeed M.A., Clarkson T.W., Doherty R.A., and Greenwood M.R. Studies of infants postnatally exposed to methylmercury. J Pediatr 1974: 85: 81–84.
Amin-Zaki L., Elhassani S.B., Majeed M.A., Clarkson T.W., Doherty R.A., and Greenwood M.R. Methylmercury poisoning in mothers and their suckling infant. Dev Toxicol Environ Sci 1980: 8: 75–78.
Amin-Zaki L., Majeed M.A., Greenwood M.R., Elhassani S.B., Clarkson T.W., and Doherty R.A. Methylmercury poisoning in the Iraqi suckling infant: a longitudinal study over five years. J Appl Toxicol 1981: 1: 210–214.
Anderson J.W., Johnstone B.M., and Remley D.T. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr 1999: 70: 525–535.
Bakir F., Damluji S.F., Amin-Zaki L., Murtadha M., Khalidi A., Alrawi N.Y., Tikriti S., Dhahir H.I., Clarkson T.W., Smith J.C., and Doherty R.A. Methylmercury poisoning in Iraq — interuniversity report. Science 1973: 181: 230–241.
Borrell A. Pcb and Ddts in blubber of cetaceans from the Northeastern North-Atlantic. Mar Pollut Bull 1993: 26: 146–151.
Carlson S.E. Long-chain polyunsaturated fatty acids and the development of human infants. Acta Paediatr Suppl 1999: 88: 72–77.
Davidson A.N., and Dobbing J, (Eds.). Vulnerable periods in developing brain. Applied Neurochemistry. Davis, Philadelphia, 1968, pp. 287–316.
Gartner L.M., Black L.S., Eaton A.P., Lawrence R.A., Naylor A.J., Neifert M.E., Ohare D., Schanler R.J., Georgieff M., Piovanetti Y., and Queenan J. Breastfeeding and the use of human milk. Pediatrics 1997: 100: 1035–1039.
Gladen B.C., and Rogan W.J. Effects of perinatal polychlorinated-biphenyls and dichlorodiphenyl dichloroethene on later development. J Pediatr 1991: 119: 58–63.
Grandjean P., Budtz-Jorgensen E., Steuerwald U., Heinzow B., Needham L.L., Jorgensen P.J., and Weihe P. Attenuated growth of breast-fed children exposed to increased concentrations of methylmercury and polychlorinated biphenyls. FASEB J 2003: 17: U443–U457.
Grandjean P., Budtz-Jorgensen E., White R.F., Jorgensen P.J., Weihe P., Debes F., and Keiding N. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol 1999: 150: 301–305.
Grandjean P., Jorgensen P.J., and Weihe P. Human-milk as a source of methylmercury exposure in infants. Environ Health Perspect 1994: 102: 74–77.
Grandjean P., Jørgensen P.J., and Weihe P. Validity of mercury exposure biomarkers. In: Wilson S.H., Suk W.A. (Eds.). Biomarkers of Environmentally Associated Disease. CRC Press/Lewis Publishers, Boca Raton, FL, 2002, pp. 235–247.
Grandjean P., Weihe P., Burse V.W., Needham L.L., Storr-Hansen E., Heinzow B., Debes F., Murata K., Simonsen H., Ellefsen P., Budtz-Jorgensen E., Keiding N., and White R.F. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol 2001: 23: 305–317.
Grandjean P., Weihe P., Jørgensen P.J., Clarkson T., Cernichiari E., and Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health 1992: 47: 185–195.
Grandjean P., Weihe P., Needham L.L., Burse V.W., Patterson D.G., Sampson E.J., Jorgensen P.J., and Vahter M. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res 1995a: 71: 29–38.
Grandjean P., Weihe P., and White R.F. Milestone development in infants exposed to methylmercury from human-milk. Neurotoxicology 1995b: 16: 27–33.
Grandjean P., Weihe P., White R.F., Debes F., Araki S., Yokoyama K., Murata K., Sorensen N., Dahl R., and Jorgensen P.J. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997: 19: 417–428.
Jacobson J.L., Jacobson S.W., and Humphrey H.E.B. Effects of in utero exposure to polychlorinated-biphenyls and related contaminants on cognitive-functioning in young-children. J Pediatr 1990: 116: 38–45.
Jensen A.A., and Slorach S.A. Chemical Contaminants in Human Milk. CRC Press, Boston, MA, 1991, p. 298.
Myers G.J., Davidson P.W., Shamlaye C.F., Axtell C.D., Cernichiari E., Choisy O., Choi A., Cox C., and Clarkson T.W. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology 1997: 18: 819–829.
Raven J. Standard Progressive Matrices. HK Levis, London, 1958.
Reynolds W.A., and Pitkin R.M. Transplacental passage of methylmercury and its uptake by primate fetal tissues. Proc Soc Exp Biol Med 1975: 148: 523–526.
Rowland I.R. Factors affecting metabolic-activity of the intestinal microflora. Drug Metab Rev 1988: 19: 243–261.
Rowland I.R., Robinson R.D., and Doherty R.A. Effects of diet on mercury metabolism and excretion in mice given methylmercury — role of gut flora. Arch Environ Health 1984: 39: 401–408.
Saarinen U.M., and Kajosaari M. Breast-feeding as prophylaxis against atopic disease — prospective follow-up-study until 17 years old. Lancet 1995: 346: 1065–1069.
World Health Assembly Resolution 25. Infant and Young Child Nutrition. World Health Organization, Geneva, 2002.
WHO. Methylmercury (Environmental Health Criteria 101). World Health Organization, Geneva, 1990.
Yamamoto R., and Suzuki T. Effects of artificial hair-waving on hair mercury values. Int Arch Occup Environ Health 1978: 42: 1–9.
Yasutake A., Matsumoto M., Yamaguchi M., and Hachiya N. Current hair mercury levels in Japanese: survey in five districts. Tohuku J Exp Med 2003: 199: 161–169.
Acknowledgements
This work was supported by grants from the US National Institute of Environmental Health Sciences (ES06112), the European Commission (Environmental Research Programme), the Danish Medical Research Council (Grant No. SV1736), and the Dannin Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jensen, T., Grandjean, P., Jørgensen, E. et al. Effects of breast feeding on neuropsychological development in a community with methylmercury exposure from seafood. J Expo Sci Environ Epidemiol 15, 423–430 (2005). https://doi.org/10.1038/sj.jea.7500420
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jea.7500420
Keywords
This article is cited by
-
A Metalloproteomics Study on the Association of Mercury With Breast Milk in Samples From Lactating Women in the Amazon Region of Brazil
Archives of Environmental Contamination and Toxicology (2015)
-
Mercury Concentration in Breast Milk and Infant Exposure Assessment During the First 90 Days of Lactation in a Midwestern Region of Brazil
Biological Trace Element Research (2013)
-
The Importance of Children’s Environmental Health for the Field of Maternal and Child Health: A Wake-Up Call
Maternal and Child Health Journal (2010)