Abstract
Thiazolyl peptides are a class of natural products with potent Gram-positive antibacterial activities. Lack of aqueous solubility precluded this class of compounds from advancing to clinical evaluations. Nocathiacins and thiazomycins are sub-classes of thiazolyl peptides that are endowed with structural features amenable for chemical modifications. Semi-synthetic modifications of nocathiacin led to a series of analogs with improved water solubility, while retaining potency and antibacterial spectrum. We studied the activities of a selection of two natural products (nocathiacin and thiazomycin) as well as seven polar semi-synthetic analogs against twenty clinical strains of Mycobacterium tuberculosis with MDR phenotypes. Two compounds show useful activity against H37Rv strain with MIC values ⩽1 μM, two (⩽0.5 μm) and three (⩽10 μm). These two derivatives showed MIC values ⩽2.5 μm against most of the 20 MDR strains regardless their resistance profile. Specifically, these lack cross-resistance to rifampicin, isoniazid and moxifloxacin.
Similar content being viewed by others
Main
Nocathiacins and thiazomycins are members of the thiazolyl peptide class of natural product antibiotics produced by Amycolatopsis fastidiosa.1, 2, 3, 4 These compounds are highly potent broad-spectrum Gram-positive agents.5 Importantly, they exhibit comparable activity against drug-resistant strains of Gram-positive pathogens including MRSA and show potent in vivo activity when dosed by parenteral administration.5 The natural thiazolyl peptides are highly lipophilic and poorly soluble in aqueous media severely limiting their potential as therapeutic agents. Syntheses of a series of polar analogs have been reported by targeted semi-synthetic efforts with retention of potency and Gram-positive antibacterial spectrum.6, 7 Nocathiacin I was reported to show potent activity against the laboratory strain of Mycobacterium tuberculosis H37Rv.8 Tuberculosis caused by drug-resistant M. tuberculosis strains continues to spread unabated in many regions of the world, which lack effective treatment options.9 The exceptional potency against Gram-positive bacteria, lack of cross-resistance to known antibiotics, coupled with the ready availability of the natural and semi-synthetic compounds in our sample collection, provided the impetus to investigate a series of these compounds against a panel of genetically defined clinical strains of M. tuberculosis with a variety of drug-resistance profiles.
We selected nine compounds for this study. They include the two parent natural products plus seven semi-synthetic water-soluble derivatives of nocathiacin I. All compounds (1–9) were tested against three strains (Table 1) of M. tuberculosis including the laboratory H37Rv strain. Two compounds (1 and 2) were available in larger amounts and were evaluated extensively against a panel of 20 clinical M. tuberculosis strains with an array of drug-resistance profiles (Table 2). Although it is well appreciated that nocathiacin I compounds are unlikely to yield drug candidates with oral efficacy, with advanced delivery technology it is feasible to administer derivatives of this class directly to the lung by inhaled routes. The activity profile and structure activity relationship of these compounds are discussed herein.
Materials and methods
Reagents and test compounds
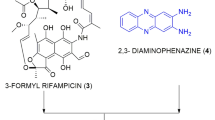
All reagents, including the antibiotic controls—isoniazid (Inh), rifampicin (Rif) and moxifloxacin hydrochloride (moxi)—were obtained from Sigma-Aldrich (St Louis, MO, USA) unless otherwise indicated. Nocathiacin I (1) and thiazomycin (4) (Figure 1) were obtained from the MRL sample repository and were originally isolated from extracts of Amycolatopsis fastidiosa.3, 10 The semi-synthetic derivatives (2, 3 and 5–9) (Figure 1) also were obtained from the MRL sample repository and were prepared as described.6, 11 The solubility of hydrochloride salts of the parent natural products 1 and 4 in 5% dextrose-water was ~0.34 mg ml−1, whereas the solubility of the semi-synthetic derivatives 2, 3, and 5–9 was >10 mg ml−1.
Sources of strains
Strains of M. tuberculosis were selected from the PHRI TB Center collection containing more than 33 000 clinical isolates and represent all nine clusters that define the M. tuberculosis phylogenetic tree (Figure 2).
MIC or growth inhibition determination
Twenty strains were sub-cultured on the 7H11 Middlebrook agar media (VWR) enriched with BBL Middlebrook OADC (Fisher), collected using cotton swab (VWR) and resuspended in phophate-buffered saline to 1.0 McFarlands standard (corresponds to 1 × 107 CFU per ml). Ten microlitre of three dilutions (1:10 in phophate-buffered saline) were plated (corresponding to 102, 103 and 104 CFU) onto one quadrant of X-plates (VWR) containing 2.5, 25 or 250 (or 1, 10 or 100) μm of a given compound (dissolved in DMSO). Rif, Inh and moxi were used as control anti-tuberculosis drugs at concentration 0.1, 1.0, 10; 0.2, 1.0, 10 and 0.2, 2.0 and 10 μm. Viability of clinical isolates was determined on the control plates with no drug. Plates were incubated at 37 °C for 14–21 days.
Result and discussion
Thiazolyl peptide classes of compounds were discovered as early as the 1950s and are well studied. They are highly potent antibacterial agents.12 However, none of the compounds from this class could be developed as a clinical agent due to extremely poor water solubility. Nocathiacins and thiazomycins are recent entries in the thiazolyl peptide class with potent activity.10, 13 They are endowed with structural features that are reasonably amenable to chemical modifications; therefore, we undertook semi-synthetic modification of the most abundant of the natural products, nocathiacin I (1) and thiazomycin (4) (Figure 1), leading to the synthesis of a series of highly potent, broad-spectrum Gram-positive agents with improved water solubility and in vivo activity.6, 7 Seven structurally diverse analogs (2–3, 5–9, Figure 1), with modifications on the pyridyl hydroxyl group and/or replacement of the dehydroalanine amide with polar substituents, were selected for this study. For initial biological evaluations, three representative M. tuberculosis strains were selected to assess potency, spectrum and structure activity relationship (SAR). The test strains included the laboratory strain H37Rv, and the two most successful M. tuberculosis clones from New York City patients since the re-emergence in the early 1990s; the highly multidrug-resistant ‘W’ strain that was a nosocomial pathogen across numerous hospitals and the pan-susceptible community acquired ‘C’ strain that spread among the homeless.14, 15 The three strains were challenged against compounds 1 and 2 at concentrations of 2.5, 25 and 250 μm, whereas compounds 3–9, for which limited quantities were available, were tested at 1, 10 and 100 μm. The inhibitory activities against M. tuberculosis strains are presented in Table 1.
The natural product nocathiacin I showed potent activity against all three strains with MIC of ⩽2.5 μm. Thiazomycin was 4-fold less potent (MIC ⩽10 μm) against the H37Rv strain and ~40-fold less active against the other two strains. Substitution of nocathiacin’s dehydroalanine with a morpholine propyl amide produced analog 2 with fully retained in vitro activity and significantly improved water solubility (0.34 vs >10 mg ml−1).6 Substitution of the pyridyl OH of 2 with a methyl ether produced compound 3 with further improved potency. Substitutions of dehydroalanine with other heterocycles (5–9) diminished potency by more than fourfold with exception of 1-methyl-pyrazolyl-methyl amide (6), which showed an improved MIC (~1 μm) against H37Rv but diminished activity against the other two strains.
Of the three best derivatives (1–3), 1 and 2 were immediately available in larger amounts for testing and were further evaluated at 2.5, 25 and 250 μm against a series of eighteen additional clinical M. tuberculosis strains with varied drug susceptible and resistant profiles. The strains were selected on the basis of both their resistance profile and their genetic diversity. The phylogenetic relationships of the test strains are mapped onto the tree in Figure 2. On the basis of comparative SNP analysis, the species are divided into three principal genetic groups and further distinguished into nine genetic clusters16, 17 The MIC data for these two compounds are presented in Table 2. In general, both analogs showed potent activity with an MIC of ⩽2.5 μm against most strains, whereas only for three strains (11 677, 13 923 and 10 975) noted reduced potency (MIC >2.5 μm). It is important to note that these strains are genetically distinct: two are pan-susceptible and one is multidrug-resistant. This finding, along with the fact that MDR strains from different genetic clusters had an MIC of ⩽2.5 μm, provides good evidence that compounds 1 and 2 do not show cross-resistance to isoniazid, rifampin and moxifloxacin and its activity has no obvious genetic restrictions.
These thiazolyl peptides show potent activity against M. tuberculosis regardless of resistance profile, which makes them interesting candidates for potential development. The solubility characteristics of the compounds does not have a critical role in their potency, which provides avenues for two different approaches for potential development (for example, soluble vs less-soluble compound) of inhaled products. Both of these analogs have shown potent systemic in vivo activity with sub mg kg−1 ED99 against murine Staphylococcus aureus infection models.5, 6 Further studies are needed to validate an in vivo effect of these compounds against M. tuberculosis and to assess whether they are worthy of further development by alternative inhaled dosing paradigms for serious life threatening tuberculosis.
References
Jayasuriya, H. et al. Isolation and structure of platencin: a novel FabH and FabF Dual inhibitor with potent broad spectrum antibiotic activity produced by Streptomyces platensis MA7339. Angew. Chem. Int. Ed. Engl. 46, 4684–4688 (2007).
Zhang, C. et al. Isolation, structure and antibacterial activity of Thiazomycin A, a potent thiazolyl peptide antibiotic from Amycolatopsis fastidiosa. Bioorg. Med. Chem. 16, 8818–8823 (2008).
Zhang, C. et al. Thiazomycins, thiazolyl peptide antibiotics from Amycolatopsis fastidiosa. J. Nat. Prod. 72, 841–847 (2009).
Singh, S. B. et al. Occurrence, distribution, dereplication and efficient discovery of thiazolyl peptides by sensitive-resistant pair screening. J. Antibiot. (Tokyo) 66, 599–607 (2013).
Singh, S. B. et al. Antibacterial evaluations of thiazomycin- a potent thiazolyl peptide antibiotic from Amycolatopsis fastidiosa. J. Antibiot. (Tokyo) 60, 565–571 (2007).
Xu, L. et al. Nocathiacin analogs: synthesis and antibacterial activity of novel water-soluble amides. Bioorg. Med. Chem. Lett. 19, 3531–3535 (2009).
Xu, L. et al. Synthesis and antibacterial activity of novel water-soluble nocathiacin analogs. Bioorg. Med. Chem. Lett. 23, 366–369 (2013).
Pucci, M. J. et al. Antimicrobial evaluation of nocathiacins, a thiazole peptide class of antibiotics. Antimicrob. Agents Chemother. 48, 3697–3701 (2004).
Abubakar, I. et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect. Dis. 13, 529–539 (2013).
Jayasuriya, H. et al. Isolation and structure elucidation of thiazomycin- a potent thiazolyl peptide antibiotic from Amycolatopsis fastidiosa. J. Antibiot. (Tokyo) 60, 554–564 (2007).
Debenham, S. D. et al Antibiotic compounds WO2007127135 (2007).
Bagley, M. C., Dale, J. W., Merritt, E. A. & Xiong, X. Thiopeptide antibiotics. Chem. Rev. 105, 685–714 (2005).
Li, W. et al. Nocathiacins, new thiazolyl peptide antibiotics from Nocardia sp. I. Taxonomy, fermentation and biological activities. J. Antibiot. (Tokyo) 56, 226–231 (2003).
Munsiff, S.S. et al. Persistence of a highly resistant strain of tuberculosis in New York City during 1990-1999. J. Infect. Dis. 188, 356–363 (2003).
Macaraig, M et al. Strain-specific differences in two large Mycobacterium tuberculosis genotype clusters in isolates collected from homeless patients in New York City from 2001 to 2004. J. Clin. Microbiol. 44, 2890–2896 (2006).
Gutacker, M. M. et al. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics 162, 1533–1543 (2002).
Mathema, B., Kurepina, N. E., Bifani, P. J. & Kreiswirth, B. N. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19, 658–685 (2006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The work described in this paper was conducted and supported by Merck & Co., Inc., a for-profit public company dedicated to discovery, development, manufacturing and sale of antibiotics and other drugs. The remaining authors declare no conflict of interest.
Additional information
This article is dedicated to Professor Satoshi Ōmura for discovery of a large number of natural products that continue to improve human lives.
Rights and permissions
About this article
Cite this article
Singh, S., Xu, L., Meinke, P. et al. Thiazomycin, nocathiacin and analogs show strong activity against clinical strains of drug-resistant Mycobacterium tuberculosis. J Antibiot 70, 671–674 (2017). https://doi.org/10.1038/ja.2016.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.165
This article is cited by
-
Identification of peptides from honeybee gut symbionts as potential antimicrobial agents against Melissococcus plutonius
Nature Communications (2023)
-
Natural thiopeptides as a privileged scaffold for drug discovery and therapeutic development
Medicinal Chemistry Research (2019)