Abstract
Screening for circumventors of β-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA) led us to find 17 novel antibiotics, naphthacemycins A1–A11, B1–B4 and C1–C2. The naphthacemycins were isolated from a cultured broth of Streptomyces sp. KB-3346-5 by repeated silica gel column chromatography and HPLC. Naphthacemycins enhanced imipenem activity 100–500 times against MRSA at 0.5 μg ml−1, and naphthacemycins A4–A11 themselves showed MIC50 values of 1–4 μg ml−1 against 22 MRSA strains.
Similar content being viewed by others
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a causative microorganism of opportunistic infection that harms individuals in medical facilities who have compromised immune systems. Recently, it has also been gaining in significance as a cause of serious community-acquired infections among healthy people.1 Although there are a few antibiotics used to combat MRSA (for example, vancomycin, teicoplanin, arbekacin, linezolid, daptomycin, tigecycline, ceftaroline, dalbavancin, oritavancin and tedizolid) microorganisms with resistance to some of them are increasingly being reported. In the course of screening for new antibiotics active against MRSA, we have recently found biverlactones, which are capable of circumventing arbekacin resistance in MRSA. They are thought to inhibit aminoglycoside-modifying enzymes.2 We have also reported that cyslabdan enhances imipenem activity against MRSA.3, 4 Our continuous study to find microbial metabolites, like cyslabdan, that circumvent β-lactam resistance of MRSA, led us to discover further novel compounds, the naphthacemycins A1–A11 (1–11), B1–B4 (12–15) and C1–C2 (16–17) (Figure 1), reported as KB-3346-5 substances in the patent by our group5, isolated from a culture broth of Streptomyces sp. KB-3346-5. In this report, we describe the taxonomy of the producing strain, as well as the fermentation, isolation and antibacterial activities of these naphthacemycin compounds.
Results and Discussion
Taxonomy of the producing organism
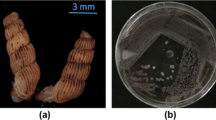
Strain KB-3346-5 was originally isolated from a soil sample collected in Okinawa Prefecture, Japan. The vegetative mycelia developed well on yeast extract–malt extract agar and nutrient agar, and the color was brown. The aerial mycelia were produced abundantly on yeast extract–malt extract agar, and the aerial mass color showed white to yellow. The mature spore chains were spiral and each had more than 20 spores per chain. The spores were cylindrical in shape, 0.5–0.6 × 0.7–0.8 mm in size and had a smooth surface (Figure 2). The isomer of diaminopimelic acid in whole-cell hydrolysates was LL-form. Major menaquinones were MK-9 (H6) and MK-9 (H8). Based on the taxonomic properties above, the microorganism was considered to belong to the genus Streptomyces6 and was named Streptomyces sp. KB-3346-5. The strain was deposited at the International Patent Organism Depositary, National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan, as FERM BP-10834.
Fermentation and isolation
A loop of cells of strain KB-3346-5 on the agar slant was inoculated into each of five test tubes containing 10 ml of a seed medium and shaken at 27 °C for 3 days. The seed broth (10 ml) was inoculated into each of five 500-ml Erlenmeyer flasks containing 100 ml of the seed medium and incubated on a rotary shaker at 27 °C for 3 days. The second seed broth (10 ml) was inoculated into each of fifty 500-ml Erlenmeyer flasks containing 100 ml of a production medium, and the fermentation was carried out on a rotary shaker at 28 °C for 8 days. The production of naphthacemycins began at 3–4 days and reached nearly maximum at 6 days (data not shown).
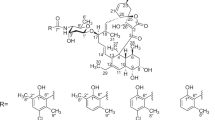
The cultured broth was extracted with acetone, and the extract was further extracted with ethyl acetate. The ethyl acetate extract was purified by silica gel column chromatography twice and HPLC to yield naphthacemycins A1–A11 (1–11), B1–B4 (12–15) and C1–C2 (16–17). Their structures were elucidated by NMR experiments and X-ray analysis, as reported elsewhere.7 Naphthacemycins displayed unique skeletons as natural products, consisting of a naphthacene ring monosubstituted with a phenyl residue at C-7 (Figure 1).
Biological activities
The circumvention of β-lactam resistance in MRSA was measured by enhancement of imipenem activity against MRSA. Naphthacemycins alone showed MIC values of 8–64 μg ml−1 against clinically isolated MRSA strain K24. In the presence of each 0.5 μg ml−1 of naphthacemycins, the MIC of imipenem was evaluated by the liquid dilution method. Without naphthacemycins, the MIC of imipenem was 32 μg ml−1. As shown in Table 1, addition of naphthacemycins reduced the MIC of imipenem to between 0.06 and 0.25 μg ml−1, yielding 128–512 times enhancement of imipenem activity. Among naphthacemycins, 3, 6, 7 and 13–15 showed 512 times enhancement.
The circumvention activity was further evaluated by a larger panel of staphylococci for sensitivity testing of imipenem activity using 22 clinically isolated MRSA and five MSSA (methicillin-sensitive S. aureus) strains. Concentrations of naphthacemycins, of 0.2 μg ml−1 (for compounds 4–11) and 1 μg ml−1 (for compounds 1–3 and 12–17), did not inhibit the growth of tested bacteria. The ranges of MIC values and the values of MIC50 of the MRSA and MSSA strains are shown in Table 2. Most naphthacemycins reduced MIC50 values of imipenem and 2 reduced the value 32-fold. However, naphthacemycins had no effect on imipenem activity against MSSA strains.
Thus, naphthacemycins circumvent imipenem resistance in MRSA, similar to cyslabdan, which we have reported previously,4 but the enhancement ratio of cyslabdan, was much higher (128 times at population analysis) than with the naphthacemycins. It is interesting that naphthacemycins enhanced imipenem activity at the concentration lower than that of cyslabdan, although the enhancement ratio was lower than cyslabdan. Therefore, antibacterial activity of naphthacemycins alone against MRSA and MSSA strains were evaluated by a larger panel of staphylococci for sensitivity testing (Table 3). Naphthacemycins other than 1, 12, 16 and 17 showed equal antibacterial activity against both MRSA and MSSA strains, and the MIC50 of 11 was 1 μg ml−1 and that of 5–8 and 10 was 2 μg ml−1, comparable to vancomycin.
The antibacterial activity of 8 and 9 (which were available in suitable quantities) against various S. aureus and a range of other bacteria was evaluated (Table 4). Compounds 8 and 9 showed antibacterial activity against Gram-positive bacteria. Their anti-MRSA activity was comparable to vancomycin, and they also inhibited the growth of linezolid-resistant MRSA. Moreover, they showed good inhibition against vancomycin-resistant Enterococcus faecalis and E. faecium. Acute toxicity of 8 and 9 against mice was not observed at 100 mg kg−1 (s.c.).
The naphthacemycin A series has a unique skeleton of 7-phenylnaphthacene-5,6,11(12H)-trione. Most structurally related compounds, tetarimycin A (18, Figure 3)8 and fasamycins (19, 20)9, were recently reported by Brady and co-workers as antibacterial agents. The antibacterial activity mechanism was identified as inhibition of FabF, one of the enzymes involved in type II fatty acid biosynthesis.10 Therefore, biological properties of naphthacemycins might be related to FabF inhibition, though we have not tested whether they could show inhibitory activity against FabF and type II fatty acid biosynthesis or not yet. Tetracycline (21) produced by Streptomyces spp. also has a naphthacene skeleton, but it is much more saturated and has a kink between rings A and B, which is believed to be involved in its binding to ribosomes.11 Chelocardin (22), produced by Nocardia sulphurea, has a much more planar skeleton.12 Its bactericidal activity is not caused by protein synthesis inhibition but is believed to be due to membrane disruption.13 Tetracenomycin C (23), produced by S. glaucescens, is an another type of naphthacene antibiotic.14, 15 It binds to DNA and mainly inhibits the growth of actinobacteria. EA-371α (24), produced by Streptomyces sp., was isolated as an inhibitor of the MexAB-OprM efflux pump, which is involved in intrinsic antibiotic resistance of Pseudomonas aeruginosa.16 Though its skeleton is 8-oxobenzo[a]naphthacene, it has a 1,3-dihydroxy-10,10-dimethylanthrone unit, similar to the naphthacemycins. Its desulfonated analog, benastatin A (25), is active against Gram-positive bacteria.17 Bischloroanthrabenzoxocinone (26), produced by Streptomyces sp., also has the same unit.18, 19 It also inhibits bacterial type II fatty acid biosynthesis and shows antibacterial activity against Gram-positive bacteria. The currently unknown mode of action of naphthacemycins may be similar to that of one of the above compounds.
Methods
Taxonomy of the producing organism
The International Streptomyces Project media recommended by Shirling and Gottlieb20 as well as media recommended by Waksman21 were used to investigate the cultural characteristics. Cultures were observed after incubation for 2 weeks at 28 °C. The morphological properties were observed using a scanning electron microscope JSM-5600 (JEOL, Akishima, Japan). Isomers of diaminopimelic acid in whole-cell hydrolysates were elucidated by TLC22 and menaquinones were analyzed by HPLC.23
Media
The agar slant medium for the stock culture of the strain KB-3346-5 consisted of starch (Kanto Chemical Co., Tokyo, Japan) 1.0%, NZ amine (Wako Pure Chemical Industries, Osaka, Japan) 0.3%, yeast extract (Oriental Yeast Co., Tokyo, Japan) 0.1%, meat extract (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan) 0.1%, CaCO3 0.3% and agar (Shimizu Shokuhin Kaisha, Shizuoka, Japan) 1.5%, adjusted to pH 7.0 before sterilization. The seed medium consisted of starch 2.4%, glucose 0.1%, peptone (Kyokuto Pharmaceutical Industrial Co.) 0.3%, yeast extract 0.5%, meat extract 0.3% and CaCO3 0.4%, adjusted to pH 7.0 before sterilization. The production medium consisted of glucose 0.5%, corn steep powder (Marcor Development Co., Carlstadt, NJ, USA) 0.5%, oatmeal (Nippon Food Manufacturer, Sapporo, Japan) 1.0%, Pharmamedia (Traders Protein, Lubbock, TX, USA) 1.0%, K2HPO4 0.5%, MgSO4·7H2O 0.5%, FeSO4·7H2O 0.0001%, MnCl2·4H2O 0.0001%, ZnSO4·7H2O 0.0001%, CuSO4·5H2O 0.0001% and CoCl2·6H2O 0.0001%, adjusted to pH 7.0 before sterilization.
Isolation
A mixture of the cultured broth (5 l) and the same volume of acetone was shaken for 30 min. Acetone in the solution was removed by evaporation and the remaining water solution was partitioned with 10 l of ethyl acetate. The ethyl acetate extract (6.73 g) was applied to a silica gel column (220 g of silica gel 60 (0.063–0.200 mm), Merck KGaA, Darmstadt, Germany), washed with CHCl3 and eluted with CHCl3–MeOH (100:1, 100:2 and 100:10). Active fractions eluted with CHCl3–MeOH (100:1) and CHCl3–MeOH (100:10) were concentrated to yield crude materials I (4.37 g) and II (0.83 g), respectively.
A total of 1.0 g of the crude material I was used for the further purification. It was applied to a silica gel column (30 g of silica gel 60 (0.040–0.063 mm), Merck KGaA) and eluted with CHCl3–MeOH (100:0, 100:1 and 100:2). Naphthacemycins were eluted at 100:0 to 100:2 ratio solutions to yield crude materials III (472 mg) and IV (69.2 mg). The crude material III was separated by ODS HPLC (column, Pegasil ODS, ϕ20 × 250 mm, Senshu Scientific Co., Tokyo, Japan; mobile phase, 60% CH3CN; flow rate, 8 ml min−1). Crude material V (295 mg) and naphthacemycins A10 (10, 14.7 mg), A11 (11, 55.7 mg) and C2 (17, 9.2 mg) were eluted at 43, 49, 51 and 55 min, respectively. The crude material V was further purified by ODS HPLC (column, Pegasil ODS, ϕ20 × 250 mm; mobile phase, 85% CH3CN; flow rate, 8 ml min−1) to yield naphthacemycins A8 (8, 106 mg) and A9 (9, 175 mg) at the eluate of 23 and 27 min, respectively. Crude material IV was purified by Pegasil ODS HPLC using 55% CH3CN (flow rate, 8 ml min−1) to yield naphthacemycins A5 (5, 26.4 mg), A6 (6, 11.5 mg) and A7 (7, 14.3 mg) at the retention times of 25, 36 and 38 min, respectively.
The crude material II was applied to a silica gel column (25 g of silica gel 60 (0.040–0.063 mm), Merck KGaA) and eluted with CHCl3–MeOH (100:2 and 100:10). Naphthacemycins eluted at 100:2 and 100:10 ratios were collected to yield crude materials VI (170 mg) and VII (251 mg), respectively. The crude material VI was separated by Pegasil ODS HPLC using 75% MeOH (flow rate, 8 ml min−1), and naphthacemycins A2 (2, 13.9 mg), B3 (14, 10.0 mg) and B4 (15, 30.5 mg) and crude materials VIII (20.2 mg) and IX (61.2 mg) were eluted at 17, 19, 27, 22 and 36 min, respectively. The crude material VIII was applied to Pegasil ODS HPLC using 55% CH3CN (flow rate, 8 ml min−1), and the eluted peak of 18 min was collected to yield naphthacemycin C1 (16, 14.2 mg). The crude material IX was further purified by Pegasil ODS HPLC using 55% CH3CN (flow rate, 8 ml min−1) to yield naphthacemycins A3 (3, 21.5 mg) and A4 (4, 32.8 mg) at the eluate of 21 and 24 min, respectively. The crude material VII was separated by Pegasil ODS HPLC using 50% CH3CN (flow rate, 8 ml min−1), and crude material X (29.9 mg) and naphthacemycin B2 (13, 89.6 mg) were eluted at 12 and 19 min, respectively. The crude material X was further purified by Pegasil ODS HPLC using 65% MeOH (flow rate, 7 ml min−1) to yield naphthacemycins B1 (10, 10.0 mg) and A1 (1, 14.2 mg) at the eluate of 36 and 44 min, respectively.
Assay of antibacterial activity
Measurement of anti-MRSA activity of imipenem enhanced by naphthacemycins were carried out by the liquid microdilution method24 using MRSA strain K24, as reported previously.4
A larger panel for sensitivity testing of clinically isolated MRSA (22 strains) and drug susceptible (MSSA) organisms (5 strains) were carried out by the agar dilution method25 in the presence of imipenem and/or naphthacemycins. An overnight culture of MRSA and MSSA strains was diluted with fresh medium to the appropriate bacterial density and spread onto a plate of Difco Mueller Hinton Agar (Becton, Dickinson and Company, Sparks, MD, USA) containing serial twofold dilutions of imipenem or naphthacemycins. To measure imipenem activity enhancement, 0.2 or 1 μg ml−1 of naphthacemycins was added with imipenem. To measure naphthacemycins activity, naphthacemycins alone were added. The plates were incubated at 37 °C for 20 h. MIC50 was defined as the concentration at which 50% of the strains could not grow.
Antibacterial activity of naphthacemycins against a variety of bacteria were measured by the agar dilution method.25
References
Diep, B. A. & Otto, M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16, 361–369 (2008).
Iwatsuki, M. et al. Biverlactones A-D, new circumventors of arbekacin resistance in MRSA, produced by Penicillium sp. FKI-4429. Tetrahedron 67, 6644–6648 (2011).
Fukumoto, A. et al. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04-0144. I. Taxonomy, fermentation, isolation and structural elucidation. J. Antibiot. 61, 1–6 (2008).
Fukumoto, A. et al. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04-0144. II. Biological activities. J. Antibiot. 61, 7–10 (2008).
Omura, S. et al. (Kitasato Institute, Japan; Kyowa Hakko Kirin Co., Ltd., Japan). KB-3346-5 substances, their fermentative manufacture, and antibacterial agents containing them. Jpn. Kokai Tokkyo Koho, JP2009046404A (2009).
Williams, S. T., Goodfellow, M. & Alderson, G. in: Bergey's Manual of Systematic Bacteriology, Vol. 4 (eds Wiliams, S. T. et al. 2452–2492 (Williams & Wilkins, Baltimore, MD, USA, 1989).
Fukumoto, A. et al Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. II. structure elucidation. J. Antibiot. (doi:10.1038/ja.2017.29).
Kallifidas, D., Kang, H.-S. & Brady, S. F. Tetarimycin A, an MRSA-active antibiotic identified through induced expression of environmental DNA gene clusters. J. Am. Chem. Soc. 134, 19552–19555 (2012).
Feng, Z., Kallifidas, D. & Brady, S. F. Functional analysis of environmental DNA-derived type II polyketide synthases reveals structurally diverse secondary metabolites. Proc. Natl Acad. USA 108, 12629–12634 (2011).
Feng, Z., Chakraborty, D., Dewell, S. B., Reddy, B. V. B. & Brady, S. F. Environmental DNA-encoded antibiotics fasamycins A and B inhibit FabF in type II fatty acid biosynthesis. J. Am. Chem. Soc. 134, 2981–2987 (2012).
Thaker, M., Spanogiannopoulos, P. & Wright, G. D. The tetracycline resistome. Cell Mol. Life Sci. 67, 419–431 (2010).
Oliver, T. J., Prokop, J. F., Bower, R. R. & Otto, R. H. Chelocardin, a new broad-spectrum antibiotic. I. Discovery and biological properties. Antimicrob. Agents Chemother. 1962, 583–591 (1963).
Chopra, I. Tetracycline analogs whose primary target is not the bacterial ribosome. Antimicrob. Agents Chemother. 38, 637–640 (1994).
Weber, W., Zähner, H., Siebers, J., Schröder, K. & Zeeck, A. Stoffwechselprodukte von Mikroorganismen. 175. Mitteilung. Tetracenomycin C. Arch. Microbiol. 121, 111–116 (1979).
Weber, W., Zähner, H., Siebers, J., Schröder, K. & Zeeck, A. in: Actinomycetes. Zbl. Bakt. Suppl. 11 (eds Schaal, K. P. & Pulverer, G.) 465–468 (Gustav Fischer Verlag, Stuttgart, 1981).
Lee, M. D. et al. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco 56, 81–85 (2001).
Aoyagi, T. et al. Benastatins A and B, new inhibitors of glutathione S-transferase, produced by Streptomyces sp. M1384-DF12. I. Taxonomy, production, isolation, physico-chemical properties and biological activities. J. Antibiot. 45, 1385–1390 (1992).
Kodali, S. et al. Determination of selectivity and efficacy of fatty acid synthesis inhibitors. J. Biol. Chem. 280, 1669–1677 (2005).
Herath, K. B. et al. Anthrabenzoxocinones from Streptomyces sp. as liver X receptor ligands and antibacterial agents. J. Nat. Prod. 68, 1437–1440 (2005).
Shiring, E. B. & Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 16, 313–340 (1966).
Waksman, S. A. (ed.) In The Actinomycetes Vol.2, (Williams & Wilkins, Baltimore, (1961).
Hasegawa, T., Takizawa, M. & Tanida, S. A rapid analysis for chemical grouping of aerobic actinomycetes. J. Gen. Appl. Microbiol. 29, 319–322 (1982).
Collins, M. D., Pirouz, T., Goodfellow, M. & Minnikin, D. E. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 100, 221–230 (1977).
Japanese Society of Chemotherapy Report of the committee for Japanese standards for antimicrobial susceptibility testing for bacteria. Chemotherapy 38, 102–105 (1990).
Nagayama, A. et al. Final report from the Committee on Antimicrobial Susceptibility Testing, Japanese Society of Chemotherapy, on the agar dilution method (2007). J. Infect. Chemother. 14, 383–392 (2008).
Acknowledgements
This work was supported in part by a grant of the twenty-first century COE Program, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fukumoto, A., Kim, YP., Matsumoto, A. et al. Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. I. The taxonomy of the producing strain, and the fermentation, isolation and antibacterial activities. J Antibiot 70, 562–567 (2017). https://doi.org/10.1038/ja.2017.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.28
This article is cited by
-
Streptovertimycins A–H, new fasamycin-type antibiotics produced by a soil-derived Streptomyces morookaense strain
The Journal of Antibiotics (2020)
-
Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. II. Structure elucidation
The Journal of Antibiotics (2017)