Abstract
Chemical investigations of the ethyl acetate extract of Streptomyces sp. IFM 11490 have led to the isolation of six new angucycline metabolites, named elmenols C–H (1–6), along with the previously reported elmonin (7) and elmenols A (8) and B (9). The known LS1924A (10), 6-deoxy-8-methylrabelomycin (11), tetrangulol methyl ether (12) and angucyclinone (13) were additionally identified. The structures of the isolated compounds were elucidated by means of spectroscopic methods including UV, IR, HRESIMS, and 1D and 2D NMR. Compounds 1–6 were evaluated for their abilities to overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance in human gastric adenocarcinoma (AGS) cells. Compounds 5 (10 μm) and 6 (50 μm) in combination with TRAIL showed moderate activity in sensitizing TRAIL-resistant AGS cells.
Similar content being viewed by others
Introduction
Angucyclines are a large group of naturally occurring secondary metabolites produced by actinomycetes.1, 2 They exhibit a number of biological activities such as antimicrobial, antiviral, antiplatelet and cytotoxic activities.3, 4 An increasing number of angucycline derivatives with different activities have been isolated from natural sources, predominantly Streptomyces sps.1, 5, 6 Their structures are mainly based on the tetracyclic benz[a]anthracene skeleton.7, 8 In the continuation of our search for new bioactive natural products from actinomycetes,9 we collected sea water and soil samples from different areas in Japan. Our laboratory reported the isolation of aotaphenazine10, sulfotanone11, yoropyrazone12 and different pyranonaphtaquinone derivatives13 from Streptomyces sps. These compounds exhibited the ability to overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance in human gastric adenocarcinoma (AGS) cells.
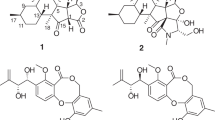
We recently investigated the crude extract of Streptomyces sp. IFM 11490 and isolated isobenzofuran derivatives, named elmonin (7) as well as elmenols A (8) and B (9).14 Further investigations of the culture of Streptomyces sp. IFM 11490 resulted in the isolation of new angucycline metabolites named elmenols C-H (1–6;Figure 1). We herein describe the isolation, structural elucidation and evaluation of the TRAIL resistance-overcoming activities of compounds 1–6 in AGS cell lines because we are interested in targeting the TRAIL-related cancer-selective apoptosis-inducing pathway using microbial natural compounds.15
Results and discussion
Streptomyces sp. IFM 11490 was isolated from a soil sample collected from Sapporo in Hokkaido, Japan. The strain was cultured in Waksman medium16 at 28 °C for 3 days on a reciprocal shaker. Working up of the crude extract resulted in the isolation of five new compounds (1–5; Figure 1) along with the known LS1924A (10)17, 6-deoxy-8-methylrabelomycin (11)18 and tetrangulol methyl ether (12).19 In addition, previously isolated elmonin (7) and elmenols A (8) and B (9)14 were detected by the LC-MS analysis. The growth of the same strain on M2 liquid medium20 under similar conditions led to the isolation of another new compound (6; Figure 1) and the known angucyclinone (10).7



Compound 1 was isolated as an optically active brown solid, [α]20D—32.3 (c 0.1, MeOH), with an assigned molecular formula of C21H22O6 based on (+)-HRESIMS at m/z 393.1326 [M+Na]+(calcd for C21H22O6Na 393.1314). The IR spectrum of 1 suggested absorption bands of OH (3396 cm−1) and carbonyl groups (1671 cm−1). The 1H NMR and 1H-1H-COSY of 1 (Table 1, Supplementary Figures S1 and S3) allowed the construction of two spin systems (Figure 2): H-5′–H-6′, ((δH 7.21 (d, J=8.4 Hz) and 6.97 (d, J=8.4 Hz)) and H-4–H-5–H-6 (δH 6.59 (d, J=7.8 Hz), 7.29 (t, J=7.8 Hz), and 6.93 (d, J=7.8 Hz)). In addition, the 1H NMR spectrum showed two methine protons ((δH 7.52 (brd) and 6.47 (d, J=2.1 Hz)), two aliphatic methylenes (δH 2.76 (d, J=16.2 Hz)/2.81 (d, J=16.2 Hz) and 3.01 (d, J=16.2 Hz)/3.11 (d, J=16.2 Hz)), two methoxy groups (δH 3.92 (s) and 3.50 (s)), and one methyl signal (δH 1.40 (s)). The 13C NMR spectrum (Supplementary Figure S2) of 1 aided by HMQC, revealed one carbonyl group (δC 201.0), five sp2 methine carbons (δC 131.9, 131.2, 122.8, 114.6 and 110.1), and seven other sp2 quaternary carbon atoms (δC 155.7, 154.5, 144.6, 134.8, 131.9, 125.0 and 125.1). In the aliphatic pattern, two oxymethines (δC 107.1 and 81.3), one oxygenated tertiary carbon (δC 71.2), two methoxy groups (δC 54.9 and 53.9), two methylenes (δC 54.4 and 44.5) and one methyl carbon (δC 27.3) were observed. The isobenzofuran moiety (unit A) in 1 was established from the interpretation of heteronuclear multiple bond correlation (HMBC) spectroscopic data. Long-range HMBC correlations from H-6/C-7a and C-4; H-5/C-7 and C-3a; and H-4/C-7a and C-6 pointed to a 1,2,3-trisubstituted benzene ring system. The HMBC couplings of oxymethines H-1/C-7 and C-3; H-3/C-7a and C-4 allowed the 1,3-dihydroisobenzofuran ring system to be confidently assigned. The position of two methoxy groups was deduced from the HMBC correlations of MeO-7/C-7 and MeO-1/C-1 (Figure 2). The proposed structure of 3,7-dihydroxy-3-methyl-1-tetralone (unit B) in 1 was confirmed by the HMBC couplings of H-6′/C-8′ and C-4′a; H-5′/C-7′ and C-8′a; H2-4′/C-8′a and C-2′ Me-3′/C-4′ and C-2′ and H2-2′/C-8′a and C-4′. It was also supported by comparing its spectral data with the known related (–)-1,4-dimethoxy-3-(3′-hydroxy-3′-methyl-1′-tetralone)-1(3H)-isobenzofuran.21 HMBC correlations from H-3 to C-7′ through 3J coupling revealed the connectivity of the 1,3-dihydroisobenzofuran fragment to C-8′ of the tetralone moiety. The relative configuration of 1 was assigned by an analysis of the NOESY spectrum and proton coupling constants (Table 1). The NOESY correlation observed between H-3/MeO-1 and H-4; H-1/MeO-7 and the small coupling constant (2.1 Hz) between H-3 and H-1 suggested the relative configuration of C-3 and C-1 (Figure 1). In the case of isobenzofuran derivatives, the coupling constant across the dual homoallylic 5JH,H is frequently larger for trans than for cis isomers.22 The structure of 1 was supported by comparisons of the spectral data with emycin E.23 On the basis of these data, compound 1 was established and named elmenol C (Figure 1).
Compound 2 was also isolated as a brown amorphous solid with positive specific rotation ([α]20D+82.1 in MeOH). The HRESIMS of 2 gave a peak at m/z 393.1316 [M+Na]+, which was consistent with the molecular formula C21H22O6, similar to compound 1. Comparisons of the UV spectra and 1D (Table 1) and 2D NMR data (Figure 2) of 2 and 1showed that both compounds had the same carbon skeleton and only differed in their stereochemistries. A careful examination of the 1H NMR data of 2 and 1 revealed differences in the chemical shifts and multiplicity between H-3 (2: δH 7.44 (s); 1: 7.52 (brd)) and H-1 (2: 6.43 (s); 1: 6.47 (d, J=2.1 Hz)). The observed J-value of ca. 0 Hz between H-1 and H-3 and the absence of a NOESY correlation between H-3 and MeO-1 led to the assignment of H-1 and H-3 in the cis-configuration. Thus, compounds 2 and 1 are epimers at C-1. Based on these results, the structure of 2 was assigned to elmenol D. Compounds 1 and 2 are new members of angucyclines and may be a product of a Baeyer–Villiger-style oxidation of tetrangulol methyl ether (12).23
Compounds 3 and 4 were obtained as brown solids. In the HPLC chromatogram, the two compounds appeared as a single peak. However, two sets of signals with 2:1 intensities in the 1H and 13C NMR spectra proposed that 3 and 4 are a mixture of two inseparable metabolites. The MW of the mixture was 350 Daltons based on (+)-ESIMS. The molecular formula was estimated to be C21H18O5 by HRESIMS m/z 373.1067 [M+Na]+(calcd for C21H18O5Na 373.1052, Δ+1.5 m.m.u.), suggesting that 3 and 4 had the same molecular compositions. The 1H and COSY NMR spectra (Table 1,Supplementary Figures S10 and S12) of 3 and 4 indicated the presence of an aromatic ABC spin system, two ortho-coupled aromatic signals, and two singlet aromatic protons. The aliphatic patterns of 3 and 4 revealed one oxygenated methine proton, two methoxy groups, and one methyl signal. The 13C NMR data (Table 2) of 3 and 4 showed seven aromatic methines, eleven quaternary carbon atoms, two methoxy groups, and one methyl carbon. An analysis of the 2D NMR spectra of 3 and 4 (Figure 2) revealed that they are closely related to elmonin (7).14 However, 3 and 4 had an additional methoxy group and the oxygenated methylene in elmonin (δH/C 5.30, 5.22/71.9)14 was replaced by a hemiacetal methine (δH/C 6.34/106.3). HMBC correlations of the methoxy protons to the hemiacetal methine carbon (C-1) revealed that 3 and 4 are a C-1 methoxylated derivative of elmonin. These results indicate that 3 and 4 are isomers with different configurations at C-1 (Figure 1). New compounds 3 and 4 were named elmenols E and F. Compounds 3 and 4 may be formed biosynthetically by oxidative ring cleavage C8/C9 of the precursor 12 followed by rearrangements.
Compound 5 was obtained as a brown solid with positive specific rotation ([α]20D+7.5 in MeOH). The molecular formula of 5 was assigned as C20H16O4 on the basis of the analysis of (+)-HRESIMS data (found m/z 343.0942 [M+Na]+, calcd for 343.0963). 1H NMR and COSY spectra (Supplementary Figures S15 and S17) showed the presence of two coupled spin systems, along with two oxymethines (δH 7.30 (s) and 6.78 (s)) that appeared as singlets. The first spin system involved two doublets (δH 6.86 (d, J=7.8 Hz) and 7.08 (d, J=7.8 Hz)) and one triplet (δH 7.21 (t, J=7.8 Hz)), which was attributed to a 1,2,3-trisubstituted aromatic system. The second spin system consisted of two ortho-coupled aromatic protons (δH 7.49 (d, J=8.7 Hz) and 6.82 (d, J=8.7 Hz)). In addition, one methoxy group (δH 3.89 (s)) and one methyl proton (δH 2.32 (s)) were observed. The 13C NMR spectrum (Supplementary Figure S16) indicated 20 carbon signals, as required by HRESIMS. There were seven sp2 aromatic methine carbons (δC 132.6, 129.9, 120.5, 120.9, 113.8, 113.4 and 112.1), nine sp2 quaternary carbons (δC 155.7, 154.7, 151.5, 147.9, 134.8, 132.7, 124.6, 120.8 and 120.4), two oxymethine signals (δC 100.8 and 80.5), one methoxy group (δC 56.7) and one methyl group (δC 22.0). In the HMBC spectrum (Supplementary Figure S19), the correlations of H-15/C-11 and C-13; H-14/C-16 and C-12; H-13/C-15 and C-11; and 12-OMe/C-12 supported the trisubstituted aromatic ring bearing a methoxy group at C-12. In addition, long-range HMBC couplings from H-2 and H-4/C-19 and 3-CH3; H-7 to C-18 and C-5; and H-6 to C-8 and C-4 supported the structure of a tetrasubstituted benzene ring fused with the substituted aryl group. HMBC correlations from 3-CH3 protons to C-4, C-3 and C-2 fixed the position of the methyl group at C-3. The important HMBC correlations of both oxymethine protons (H-17 to C-11 and C-8; and H-10 to C-16 and C-8) support the structure of 5. Compound 5 was confirmed by comparisons of NMR data with the isolated and structurally related LS1924A (10). New compound 5 was assigned to elmenol G. It is a ring-expanded angucycline with a tetracyclic ring structure, similar to emycin D.23
Compound 6 was isolated as a white amorphous solid with a positive optical rotation value ([α]20D+80.7 in MeOH). Its molecular formula was C20H15NO4 from the (-)-HRESIMS analysis m/z 332.0924 [M-H]− (calcd for C20H14NO4 332.0923). The IR spectrum of 6 showed an absorption band due to hydroxy (3380 cm−1) and carbonyl (1679 cm−1) groups. The 1H and 13C NMR spectra (Supplementary Figures S20 and S20) of 6 were similar to those of compounds 3 and 4. The major differences in the 1H NMR spectrum for 6 were the absence of the oxymethine proton and appearance of a sharp singlet NH proton [δH 9.47 (s)]. The 13C NMR spectrum of 6 lacked the oxymethine carbon that appears in 3 and 4 and instead had a carbonyl group [δC 167.9]. In addition, an upfield shift of C-3 and C-3a were observed for 6. Long-range HMBC correlations were observed from NH (δH 9.47 (s)) to C-7a, C-3, C-3a and C-1 revealing the presence of a 1-isoindolinone moiety in 6. A combination of the above results led to the structural elucidation of elmenol H (6). To the best of our knowledge, elmenol H (6) is one of the rare angucycline-containing isoindolinone moieties.
We evaluated the bioactivities of elmenols C-H (1-6) for their abilities to overcome TRAIL resistance in AGS cells. This cell line has been widely used as a model system to evaluate cancer cell apoptosis and is reported to be refractory to the induction of apoptosis by TRAIL.24 To assess the effects of compounds 1-6 on cell viability in the presence and absence of TRAIL, AGS cells were treated with the indicated agents and subjected to a fluorometric microculture cytotoxicity assay.25 Luteolin (Lut) was used as a positive control at 17.5 μm.26 The assay results (Figure 3) showed that compounds 1 and 2 did not show significant difference in the cell viability between in the presence and absence of TRAIL (100 ng ml−1), whereas compound 5 at 10 μm and compound 6 at 50 μm exhibited 25 and 30% decreases, respectively, in cell viability in the presence of TRAIL (100 ng ml−1) compared with in the absence of TRAIL. The mixture of elmenols E (3) and F (4) along with compounds 5 and 6 did not produce any significant reductions in cell viability with TRAIL.
Overall, our search for new bioactive natural products from actinomycetes yielded the terrestrial Streptomyces sp. IFM 11490, which produced six new angucycline metabolites named elmenols C-H (1-6). These new structures will enrich the structural diversity of the angucycline family of natural products. Elmenols G (5) and H (6) exhibited moderate TRAIL resistance-overcoming activities in AGS cells.
Materials and methods
General experimental procedures
NMR spectra were recorded on JEOL ECZ600 spectrometers with deuterated solvents, the chemical shifts of which were used as an internal standard. Low-resolution electrospray ionization mass spectra (ESIMS) were obtained on a Shimadzu LCMS-2020 spectrometer. High-resolution electrospray ionization mass spectra (HRESIMS) were recorded on an AccuTOF-T100LP (JEOL) mass spectrometer. IR spectra were measured on ATR (attenuated total reflection) on a JASCO FT-IR 230 spectrophotometer. UV spectra were measured on a Shimadzu UV mini-1240 spectrometer.
Identification of the bacterial strain
The identification of Streptomyces sp. IFM 11490 was performed by Professor Tohru Gonoi at the Medical Mycology Research Center, Chiba University, at which a voucher specimen is deposited with the code IFM 11490. This strain was identified on the basis of 16S rRNA gene sequences. A BLAST analysis revealed that the isolate IFM 11490 belonged to the genus Streptomyces sp. and had 98.7% similarity with the 16S rRNA of Streptomyces hyderabadensis.
Culture media
Waksman liquid medium consisting of glucose (2.0 g/100 ml), meat extract (0.5 g/100 ml), peptone (0.5 g/100 ml), dried yeast (0.3 g/100 ml), NaCl (0.5 g/100 ml) and CaCO3 (0.3 g/100 ml) was used. The composition of M2 medium was malt extract (10 g/100 ml), yeast extract (4 g/100 ml) and glucose (4 g/100 ml).
Fermentation
The isolated strain was cultivated from glycerol stock on Waksman agar plates at 28 °C for 3 days. To prepare the seed culture, chunks of a well-grown agar plate were used to inoculate 5 × 500-cm3 Sakaguchi flasks each containing 100 ml of Waksman liquid. Cultures were grown at 28 °C for 3 days with reciprocal shaking at 200 r.p.m. An aliquot of the seed culture (20 ml) was subsequently used to inoculate 32 × 3-L flasks each containing 750 ml of liquid Waksman medium. Fermentation was continued at 28 °C with shaking (200 r.p.m.) for 7 days. The strain was cultured in the same manner on M2 medium.
Extraction and isolation
The culture broth (24L) of Waksman medium was centrifuged at 6000 r.p.m. for 20 min and then extracted three times with ethyl acetate. The biomass (mycelium) was extracted three times with acetone. After the removal of acetone, the aqueous solution was extracted three times with ethyl acetate. Extracts from the water phase and biomass were combined under reduced pressure. Fractionation of the crude extract (6.90 g) using silica gel PSQ100B column chromatography with a gradient of 0–100% MeOH/CHCl3 resulted in the generation of five fractions (I-V). Fraction I (293.3 mg) was purified by Sephadex LH-20 (ϕ 25 × 240 mm) eluted with MeOH, and this was followed by preparative HPLC (YMC-Pack ODS-AM, 10 × 250 mm; eluent, 65% MeOH; flow rate, 2.0 ml min−1; UV detection at 254 nm; YMC Co., Ltd., Kyoto, Japan) to give elmenols C (1, 1.7 mg, tR 14 min) and D (2, 1.1 mg, tR 16 min) along with the known LS1924A (10, 2.9 mg). Fraction II (200 mg) was applied to an ODS column (ϕ 30 × 230 mm) eluted with MeOH to afford four sub-fractions named IIa (54.0 mg), IIb (44.4 mg), IIc (8.0 mg) and IId (97.4 mg). Sub-fraction IIa was purified by preparative HPLC (YMC-Pack ODS-AM, 10 × 250 mm; eluent, 60% MeOH; flow rate, 2.0 ml min−1; UV detection at 254 nm) to give an elmenol E (3)/F (4) mixture (2.9 mg, tR 14 min). Compound 11 (26.4 mg) was isolated as a red solid from sub-fraction IIb. Fraction III (1.20 g) was fractionated using silica gel 60N column chromatography (ϕ 30 × 230 mm) eluted with hexane/EtOAc (1:1) to give three sub-fractions named IIIa (35.0 mg), IIIb (31.8 mg) and IIIc (890 mg). Sub-fraction IIIa was also purified by HPLC (YMC-Pack ODS-AM, 10 × 250 mm; eluent, 65% MeOH; flow rate, 2.0 ml min−1; UV detection at 254 nm) to afford elmenol G (5, 1.7 mg, tR 74 min). Compound 12 (4.7 mg) was obtained as a brown solid from sub-fraction IIIb. When the strain was cultured in M2 medium (12L) and extracted under similar conditions, it gave 2.20 g of a crude extract. The extract was applied to silica gel PSQ100B column chromatography using the MeOH/CHCl3 gradient (0 to 100% MeOH) to give four fractions (VI-IX). Fraction VII (666.1 mg) was purified by the ODS column (ϕ 30 × 230 mm) using H2O: MeOH (1:1–0:1) as the eluent to yield 3.2 mg of elmenol H (6). Fraction VIII (295.1 mg) was purified by preparative HPLC using 80% MeOH in H2O (YMC-Pack ODS-AM, 10 × 250 mm; flow rate, 2.0 ml min−1) and UV detection at 254 nm to give angucyclinone (13).
Cell cultures
AGS cells were derived from the Institute of Development, Aging and Cancer, Tohoku University. Cells were cultured in RPMI-1640 medium (Wako) with 10% fetal bovine serum and maintained in a humidified incubator at 37 °C in 5% CO2/95% air.
TRAIL resistance test
TRAIL resistance was assessed by comparisons of cell viability in the presence and absence of TRAIL with TRAIL-resistant AGS cell lines. AGS cells were seeded on a 96-well culture plate (6 × 103 cells per well) in 200 μl of Roswell Park Memorial Institute medium containing 10% fetal bovine serum. Cells were incubated at 37 °C in a 5% CO2 incubator for 24 h. Test samples with or without TRAIL (100 ng ml−1) at different doses were added to each well. After a 24-h incubation, cells were washed with phosphate-buffered saline, and 200 μl of phosphate-buffered saline containing fluorescein diacetate (10 μg ml−1) was added to each well.25 The plates were then incubated at 37 °C for 1 h, and fluorescence was measured in a 96-well scanning spectrofluorometer at 538 nm with excitation at 485 nm.
Elmenol C(1)
Brown solid; [α]20D—32.3 (c 0.1, MeOH); UV (MeOH) λmax (log ɛ) 328 (2.4) and 230 (2.7) nm; IR νmax (ATR) ca. 3396, 2357, 1671, 1631, 1485 and 1278 cm−1; 1H and 13C NMR data in Tables 1 and 2; (+)-HRESIMS m/z 393.1326 [M+Na]+(calcd for C21H22O6Na 393.1314).
Elmenol D (2)
Brown solid; [α]20D+82.1 (c 0.1, MeOH); UV (MeOH) λmax (log ɛ) 328 (3.9) and 230 (4.1) nm; IR νmax (ATR) ca. 3354, 2358, 1671, 1631 and 1484 cm−1; 1H and 13C NMR data in Tables 1 and 2; (+)-HRESIMS m/z 393.1316 [M+Na]+(calcd for C21H22O6Na 393.1314).
Elmenols E (3) and F (4)
Brown solid; UV (MeOH) λmax (log ɛ) 342 (3.4), 300 (3.5), 279 (3.9), 246 (4.2) and 218 (4.4) nm; IR νmax (ATR) ca. 3335, 1614, 1488 and 1292 cm−1; 1H and 13C NMR data in Tables 1 and 2; (+)-HRESIMS m/z 373.1067 [M+Na]+(calcd for C21H18O5Na 373.1052).
Elmenol G (5)
Brown solid; [α]20D+7.5 (c 0.2, MeOH); UV (MeOH) λmax (log ɛ) 382 (3.1), 245 (3.9) and 218 (4.4) nm; IR νmax (ATR) ca. 3350, 1614, 1485 and 1278 cm−1; 1H NMR (600 MHz, acetone-d6) δ 7.49 (1H, d, J=8.7 Hz, H-6), 7.30 (1H, s, H-17) 7.21 (1H, t, J=7.8 Hz, H-14), 7.08 (1H, d, J=7.8 Hz, H-15) 7.02 (1H, d, J=1.1 Hz, H-4), 6.86 (1H, d, J=7.8 Hz, H-13), 6.82 (1H, d, J=8.7 Hz, H-7), 6.81 (1H, d, J=1.1 Hz, H-2), 6.78 (1H, s, H-10), 3.89 (3H, s, 12-OCH3), 2.32 (3H, s, 3-CH3) p.p.m; 13C NMR (150 MHz, acetone-d6) δ 155.7 (C-12), 154.7 (C-1), 151.5 (C-16), 147.9 (C-8), 134.8 (C-3), 132.7 (C-5), 132.6 (C-14), 129.9 (C-6), 124.6 (C-11), 120.5 (C-7), 120.9 (C-4), 120.8 (C-19), 120.4 (C-18), 113.8 (C-2), 113.4 (C-15), 112.1 (C-13), 100.8 (C-10), 80.5 (C-17), 56.7 (12-OCH3), 22.0 (3-CH3) p.p.m; (+)-HRESIMS m/z 343.0942 [M+Na]+(calcd for C20H16O4Na 343.0963).
Elmenol H (6)
White amorphous solid; [α]20D+80.7 (c 0.06, MeOH); UV (MeOH) λmax (log ɛ) 383 (3.2), 291 (3.8), 243 (4.4), and 212 (4.7) nm; IR νmax (ATR) ca. 3380, 2920, 1679, and 1279 cm−1; 1H and 13C NMR data in Tables 1 and 2; (-)-HRESIMS m/z 332.0924 [M-H]– (calcd for C20H14NO4 332.0923).
References
Kharel, M. K. et al. Angucyclines: biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 29, 264–325 (2012).
Rohr, J. & Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 9, 103–137 (1992).
Fan, K. et al. Evaluation of the cytotoxic activity of new jadomycin derivatives reveals the potential to improve its selectivity against tumor cells. J. Antibiot. 65, 449–452 (2012).
Abdelfattah, M. S., Kharel, M. K., Hitron, J. A., Baig, I. & Rohr, J. Moromycins A and B, isolation and structure elucidation of C-glycosylangucycline-type antibiotics from Streptomyces sp. KY002. J. Nat. Prod. 71, 1569–1573 (2008).
Song, Y. et al. Cytotoxic and antibacterial angucycline and prodigiosin analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar. Drugs 13, 1304–1316 (2015).
Brötz, E. et al. Amycomycins C and D, new angucyclines from Kitasatospora sp. Tetrahedron Lett. 55, 5771–5773 (2014).
Helaly, S. E. et al. Warkmycin, a novel angucycline antibiotic produced by Streptomyces sp. Acta 2930. J. Antibiot. 66, 669–674 (2013).
Ma, M. et al. Angucyclines and angucyclinones from Streptomyces sp. CB01913 featuring C-ring cleavage and expansion. J. Nat. Prod. 78, 2471–2480 (2015).
Ishibashi, M in Progress in the Chemistry of Organic Natural Products, Vol. 99 (eds Kinghorn, A. D., Falk, H. & Kobayashi, J.) 147–198 (Springer, 2014).
Abdelfattah, M. S., Ishikawa, N., Karmakar, U. K., Yamaku, K. & Ishibashi, M. New phenazine analogues from Streptomyces sp. IFM 11694 with TRAIL resistance-overcoming activities. J. Antibiot. 69, 446–450 (2016).
Abdelfattah, M. S., Ishikawa, N., Utpal, K. & Ishibashi, M. Sulfotanone, a new alkyl sulfonic acid derivative from Streptomyces sp. IFM 11694 with TRAIL resistance-overcoming activity. J. Nat. Med. 70, 266–270 (2016).
Abdelfattah, M. S., Toume, K. & Ishibashi, M. Yoropyrazone, a new naphthopyridazone alkaloid isolated from Streptomyces sp. IFM 11307 and evaluation of its TRAIL resistance-overcoming activity. J. Antibiot. 65, 245–248 (2012).
Abdelfattah, M. S., Toume, K. & Ishibashi, M. New pyranonaphthoquinones and phenazine alkaloid isolated from Streptomyces sp. IFM 11307 with TRAIL resistance-overcoming activity. J. Antibiot. 64, 729–734 (2011).
Yixizhuoma et al. Novel cytotoxic isobenzofuran derivatives from Streptomyces sp. IFM 11490. Tetrahedron Lett. 56, 6345–6347 (2015).
Abdelfattah, M. S., Arai, M. A. & Ishibashi, M. Bioactive secondary metabolites with unique aromatic and heterocyclic structures obtained from terrestrial actinomycetes Species. Chem. Pharm. Bull 64, 668–675 (2016).
Waksman, S. A. The Actinomycetes: Classification, Identification and Descriptions of Genera and Species Vol. 2, 61–292 (Williams & Wilkins Co., Baltimore, MD, USA, 1961).
Chen, C. et al. Isolation and characterization of LS1924A, a new analog of emycins. J. Antibiot. 65, 433–435 (2012).
Shigihara, Y. et al. 6-Deoxy-8-O-methylrabelomycin and 8-O-methyl- rabelomycin from a Streptomyces species. J. Antibiot. 41, 1260–1264 (1988).
Abdelfattah, M., Maskey, R. P., Asolkar, R. N., Grün-Wollny, I. & Laatsch, H. Seitomycin: isolation, structure elucidation and biological activity of a new angucycline antibiotic from a terrestrial Streptomycete. J. Antibiot. 56, 539–542 (2003).
Kieser, T., Bibb, M. J., Buttner, M. J., Chater, K. F. & Hopwood, D. A. Practical Streptomyces Genetics 2nd edn. (The John Innes Center Foundation, Norwich, CT, USA, 2000).
Zhang, S. M. et al. Isolation, stereochemical study, and cytotoxic activity of isobenzofuran derivatives from a marine Streptomyces sp. Chirality 27, 82–87 (2015).
Barfield, M., Spear, R. J. & Sternhell, S. Interproton spin-spin coupling across a dual path in five-membered rings. J. Am. Chem. Soc. 93, 5322–5327 (1971).
Gerlitz, M., Udvarnoki, G. & Rohr, J. Biosyntheses of novel emycins from the mutant strain Streptomyces cellulosae ssp. griseoincarnatus 1114–2. Angew. Chem. Int. Ed. 34, 1617–1621 (1995).
Srivastava, R. K. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia 3, 535–546 (2001).
Lindhagen, E., Nygren, P. & Larsson, R. The fluorometric microculture cytotoxicity assay. Nat. Protoc. 3, 1364–1369 (2008).
Horinaka, M. et al. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 24, 7180–7189 (2005).
Acknowledgements
We are thankful to Professor Tohru Gonoi (Medical Mycology Research Center, Chiba University) for the identification of Streptomyces sp. IFM 11694. This work was supported by KAKENHI Grant Nos 26305001 and 26293022 from the Japan Society for the Promotion of Science, and the Strategic Priority Research Promotion Program of Chiba University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This article is dedicated to Professor Satoshi Ōmura in celebration of his 2015 Nobel Prize.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Yixizhuoma, Ishikawa, N., Abdelfattah, M. et al. Elmenols C-H, new angucycline derivatives isolated from a culture of Streptomyces sp. IFM 11490. J Antibiot 70, 601–606 (2017). https://doi.org/10.1038/ja.2016.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.158
This article is cited by
-
Fabrication and characterization of pigmented secondary metabolites bound liposomes with improved cytotoxic activity against prostate and hepatic cancer
International Nano Letters (2022)
-
Heliomycin and tetracinomycin D: anthraquinone derivatives with histone deacetylase inhibitory activity from marine sponge-associated Streptomyces sp. SP9
3 Biotech (2018)