Abstract
The strain SCSIO 03039 was isolated from a sediment sample in the Indian Ocean and was characterized as a Nocardiopsis alba species on the basis of its 16S rRNA gene sequence. Seven diketopiperazines (DKPs), including two new DKPs nocazines D (2a) and E (2b), and five known DKPs (1, 3–6), were isolated from N. alba SCSIO 03039, along with two known compounds 2-methoxy-1,4-naphthoquinone (7) and 1-hydroxy-4-methoxy-2-naphthoic acid (8). Their structures were elucidated by mass and NMR spectroscopic analyses. The structure of methoxyneihumicin (1), previously proposed in a conference poster lacking publicly available experimental data, was validated for the first time by detailed NMR analyses and X-ray diffraction study. The two enantiomers nocazines D (2a) and E (2b) were isolated as a mixture. Compounds 3 and 4 were only known as synthetic compounds before. Methoxyneihumicin (1) exhibited in vitro cytotoxicities against MCF-7 and SF-268 with IC50 values of 4.6 and 12.7 μM, respectively, better than those of 6 (22.0 and 20.6 μM). The other compounds showed less pronounced cytotoxities against three tested human cancer cell lines and no compounds displayed antibacterial activities toward four indicator strains.
Similar content being viewed by others
Introduction
Marine actinomycetes have been proven as a prolific resource for drug discovery.1, 2, 3 However, reports of natural products derived from deep-sea (>1000 m) culturable actinomycetes are still scarce, probably because of the difficulty in accessing the deep-sea samples and the unculturability of deep-sea-derived actinomycetes.4 Our recent investigations on deep-sea-derived actinomycetes have led to the discovery of culturable new actinobacterial taxa,5, 6, 7, 8, 9 and the isolation of novel natural products, including spirotetronates,10 β-carboline and indolactam alkaloids,11 spiro-bisindole alkaloids,12 diazaanthraquinone derivatives13 and angucycline glycosides,14 which exhibit antibacterial, anti-malarial and antitumor properties. From our numerous actinomycetes derived from deep-sea sediments, the isolate SCSIO 03039, identified as a Nocardiopsis alba, was found to produce seven diketopiperazines (DKPs), including two new DKPs nocazines D (2a) and E (2b), and five known DKPs (1, 3–6). Compound 1, methoxyneihumicin, previously reported in a conference poster without available experimental data,15 was structurally validated herein for the first time by NMR analyses and the X-ray diffraction study. Compound 3 (S,Z)-3-benzylidene-6-methylpiperazine-2,5-dione and 4 (S,Z)-3-benzylidene -6-isopropylpiperazine-2,5-dione were only known as synthetic compounds before.16 The two known DKPs, (3Z,6Z)-3-(4-methoxybenzylidene)-6-(2-methylpropylidene)-piperazine-2,5-dione (5)17, 18 and XR334 (6),19 were reisolated from N. alba SCSIO 03039. In addition, another two known compounds, 2-methoxy-1,4-naphthoquinone (7)20, 21 and 1-hydroxy-4-methoxy-2-naphthoic acid (8),22 were also isolated. We reported herein the isolation, structure elucidation and biological activities of these compounds.
Results and Discussion
Taxonomy of the producing strain
The almost full 16S rRNA gene sequence (1447 bp) of the producing strain SCSIO 03039 was submitted to GenBank under the accession number JX195181, which showed the highest similarity (99.3%) to that of N. alba DSM 43377T (X97883). The phylogenetic tree generated by a neighbor-joining method clearly revealed the evolutionary relationship of the strain SCSIO 03039 with a group of Nocardiopsis species (Supplementary Figure S1). On the basis of the 16 S rRNA gene sequence analysis,23 the strain SCSIO 03039 was designated as a species of N. alba.
Isolation of compounds 1-8
The fermentation broths of 20 l culture of N. alba SCSIO 03039 were extracted with butanone and the mycelia cakes were extracted with methanol, respectively. The combined crude extracts were subjected to various chromatographic methods (such as silica gel column, Sephadex LH-20 chromatography and semipreparative HPLC) and led to the isolation of two new DKPs nocazines D (2a) and E (2b), five known DKPs (1, 3–6), along with two known compounds 2-methoxy-1,4-naphthoquinone (7) and 1-hydroxy-4-methoxy-2-naphthoic acid (8).
Structure elucidation
Compounds 5–8 (Figure 1) were found to be known compounds (3Z,6Z)-3-(4-methoxybenzylidene)-6-(2-methylpropylidene)-piperazine-2,5-dione (5),17, 18 XR334 (6),19 2-methoxy-1,4-naphthoquinone (7)20, 21 and 1-hydroxy-4-methoxy-2-naphthoic acid (8),22 respectively, by comparing their NMR data with those previously reported (Supplementary Figures S6–S9).
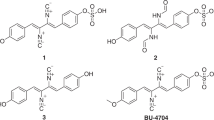
The molecular formula of compound 1 was determined to be C20H18N2O3 on the basis of high-resolution fast atom bombardment MS (HRFABMS) (m/z 335.1393 [M+H]+, calcd. for 335.1396). The 1H and 13C NMR data of 1 were very similar to those of nocazine A,24 a metabolite isolated from the marine-derived Nocardiopsis dassonvillei HR10-5. The comparison of 1H and 13C NMR data between 1 and nocazine A revealed the loss of signals for one N-Me and one methoxy group in 1. The sets of coupled 1H NMR signals at δH 7.43 (2H, ddd, J=8.0, 7.5, 1.8 HZ), 7.37 (2H, brd, J=7.5 HZ) and 7.32 (1H, tt, J=7.8, 1.5 HZ) revealed the presence of one 1-substituted benzene in 1. HMBC correlations from H-7 to C-9/C-13 located the 1-substituted benzene at C-7 (Figure 2). Thus, these comparisons indicated that 1 differed from nocazine A by losing N-1 methyl group and C-11 methoxy group (Figure 1). The X-ray crystal diffraction analysis of 1 also supports this conclusion (Figure 3), and further determined the Z configuration of both Δ6,7 and Δ3,14 double bonds. Thus, compound 1 was identified as (3Z, 6Z)-6-benzylidene-5-methoxy-3-(4-methoxybenzylidene)-1,6-dihydropyrazin-2(3H)-one (methoxyneihumicin, Figure 1). It should be noted that the structure of 1 was previously proposed in a conference poster without publicly available experimental data.15
The molecular formula of compound 2 was determined to be C19H18N2O3 on the basis of HRFABMS (m/z 345.1215 [M+Na]+, calcd for C19H18N2O3Na 345.1215). The 1H and 13C NMR data of 2 were classified into one methoxy group (δH/δC 3.83/55.4), ten sp2 methines, four sp2 quaternary carbons, one sp2 methylene, one sp3 methine and two amidocarbonyl carbons (δC 159.9, 165.0) (Table 1). The comparison of 1D NMR data of 2 with those of nocazine C24 showed that 2 only differed from nocazine C by lacking signals for N-4 methyl group (N-1 methyl group in nocazine C). Correspondingly, C-2, C-3, C-5 and C-14 (C-5, C-6, C-2 and C-7, respectively, in nocarzine C24) shifted upfield for 4.5, 4.7, 2.5 and 5.0 p.p.m., respectively. The planar structure of 2 (Figure 1) was further confirmed by HMBC correlations from H-6 to C-2/C-5, from H-14 to C-2/C-5 and from H-7 to C-5/C-6 (Figure 2). The Δ3,14 double bond in 2 was deduced to be Z configuration from the relative downfield shift of H-14 (δH 6.81), in comparison with the vinyl proton chemical shifts of H-7 (δH7.03) in nocazine C,24 and H-7 (δH 6.54) in (3S,6E)-3-benzyl-6-benzylidenepiperazine-2,5-dione,25 based on previous reports that the (Z)-vinyl proton was farther downfield than the (E)-vinyl proton because of the deshielding effect of the 5-ketone in DKP compounds.24, 25, 26, 27 Consistent with the Z configuration of Δ3,14, the NOESY correlation was observed between 4-NH (δH 9.65 in DMSO-d6) and H-20 (δH 7.18 in DMSO-d6), and no NOESY correlation between 4-NH (δH 9.65 in DMSO-d6) and H-14 (δH 6.30 in DMSO-d6) was observed (Figure 2, Supplementary Figure S3). Marfey’s method was applied to determine the absolute configuration of the phenylalanine moiety in 2.28 Unexpectedly, both L- and D-phenylalanine derivatives were found in HPLC analysis of the 1-fluoro-2,4-dinitrophenyl-5-L-alnaine amide (FDAA) derivatives of the acid hydrolysates of 2, when compared with those prepared from authentic samples of both L- and D-phenylalanine (FDAA tR=11.9 min; L-phenylalanine derivative tR=14.5 min; D-phenylalanine derivative tR=15.3 min) (Supplementary Figure S10A). Further HPLC analysis revealed that the two isomers were separable on an analytical chiral column (Supplementary Figure S10B). These data indicated that 2 was actually a mixture of two enantiomers of 2a (6R, containing a D-phenylalanine) and 2b (6S, containing an L-phenylalanine) (Figure 1). However, compound 2 was only isolated in minor amounts, preventing further efforts to obtain pure 2a and 2b by preparative chiral HPLC. Thus, the structures of enantiomers 2a and 2b were determined as (R, Z)-3-benzylpiperazine-6-(4-methoxybenzylidene)-2,5-dione and (S, Z)-3-benzylpiperazine-6-(4-methoxybenzylidene)-2,5-dione, designated nocazines D (2a) and E (2b), respectively (Figure 1).
Compound 3 was obtained as white powder. The inspection of the 1H and 13C NMR data of 3 revealed the presence of one methyl group (δH/δC 1.60/21.9), six sp2 methines, one sp3 methine, two sp2 quaternary carbons and two amidocarbonyl carbons (δC 159.7, 166.2) (Table 1). The comparison of its NMR data with that of 2 revealed the existence of 3-benzylidenepiperazine-2,5-dione moiety, which were further identified by the HMBC correlations from H-7 to C-2/C-9, from H-9 to C-7/C-11 and from H-10 to C-8. In addition, the HMBC correlations from H-14 to C-5/C-6 located the methyl group (δH/δC 1.60/21.9) at C-6. Therefore, the planar structure of 3 was determined to be 3-benzylidene-6-methylpiperazine-2,5-dione (Figure 1). The Z configuration of Δ3,7 double bond in 3 was deduced from the chemical shift of H-7 (δH 6.99), similar to that of 2 (δH 6.81, H-14) (Table 1). The absolute configuration of the alanine moiety in 3 was determined as L by Marfey’s method (FDAA tR=15.9 min; L-alanine derivative tR=14.5 min; D-alanine derivative tR=16.2 min) (Supplementary Figure S11A). Therefore, the structure of compound 3 was elucidated as (S, Z)-3-benzylidene-6-methylpiperazine-2,5-dione. The compound 3 was chemically synthesized before,16 however, only 1H NMR data were available.16
The molecular formula of compound 4 was assigned to be C14H16N2O2 by HRFABMS (m/z 245.1294 [M+H]+, calcd. for C14H17N2O2 245.1290). The 1H and 13C NMR data of 4 was consistent with those of the synthetic compound 6-benzylidene-3-isopropylpiperazine-2,5-dione. (Supplementary Figure S5). The Z configuration of Δ3,7 double bond in 4 could be deduced from the similarity of the chemical shift of H-7 (δH 7.00) to that of 3 (δH 6.99). The absolute configuration of the valine moiety in 4 was determined as L by Marfey’s method (FDAA tR=11.9 min; L-valine derivative tR=12.9 min; D-valine derivative tR=14.1 min) (Supplementary Figure S11B). Thus, compound 4 was elucidated as (S, Z)-3-benzylidene-6-isopropylpiperazine-2,5-dione (Figure 1).
Biological activity
Eight compounds (1–8) were evaluated for in vitro cytotoxic activities against three human cancer cell lines SF-268, MCF-7 and NCIH460, and antibacterial activities against four indicator strains Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Bacillus subtilis SCSIO BS01 and Bacillus thuringiensis SCSIO BT01. Methoxyneihumicin (1) showed moderate activities against human tumor cell lines MCF-7 (IC50 4.6 μM) and SF-268 (IC50 12.7 μM). Compound 6 (XR334), lacking an O-methyl group at C-5 (Figure 2), exhibited less cytotoxic activities than 1. However, when the Δ6,7 double bond in compound 6 was reduced to afford a mixture of 2a and 2b, the latter compounds showed little cytotoxic activities (Table 2). The DKPs 3-5 and the compounds 7 and 8 exhibited little antiproliferative activities against the three tested cancer cell lines (Table 2). And no compounds displayed antibacterial activities (MIC>128 μg ml−1) against four indicator strains.
DKPs are the smallest cyclic peptides known and exhibit a wide spectrum of biological activities with various therapeutic possibilities.29, 30 Recently, marine actinomycetes have been emerged as producers for novel DKPs, such as cyclomarazines,31 dimeric DKP naseseazines,32 neihumicins,33 nocardioazines (containing a novel bridged DKP scaffold),34 nocazines,24 and cyclo(2-hydroxy-Pro-R-Leu).35 However, none of the described producers were isolated from a deep-sea environment greater than the depth of 1000 m. In contrast, the strain N. alba SCSIO 03039 was isolated from the abyssal deep sedimental sample (−3412 m) in the Indian Ocean. In this study, we showed that this deep-sea-derived actinomycete was capable of producing seven DKPs. Nocazines D and E (2a and 2b) were new. Methoxyneihumicin (1) was structurally validated for the first time by NMR and X-ray analyses. Compounds 3 and 4, previously known only as synthetic compounds, were isolated for the first time from a natural source. Interestingly, methoxyneihumicin (1) displayed cytotoxicities against MCF-7 and SF-268 with IC50 values of 4.6 and 12.7 μM, significantly better than other DKPs isolated in this study.
Methods
General experimental procedures
Materials for column chromatography were silica gel (100–200 mesh; 300–400 mesh; Jiangyou Silica Gel Development, Inc., Yantai, China), Sephadex LH-20 (40–70 μm; Amersham Pharmacia Biotech AB, Uppsala, Sweden) and YMC*GEL ODS-A (12 nm S-50 μm; YMC Company Ltd., Kyoto, Japan). TLC (0.1–0.2 mm or 0.3–0.4 mm) was conducted with precoated glass plates (silica gel GF254, 10–40 nm; Jiangyou Silica Gel Development, Inc.). Medium-pressure liquid chromatography was performed on automatic flash chromatography (EZ Purifier III; Leisure Science Co., Ltd., Shanghai, China). ESI-MS was acquired on a MDS SCIEX API 2000 liquid chromatography/tandem MS (LC/MS/MS) instrument (Applied Biosystems, Inc., Forster, CA, USA). HRFABMS data were obtained on a quadrupole-time-of-flight MS (Waters, Milford, MA, USA). The optical rotation was recorded on a 341 Polarimeter (PerkinElmer, Inc., Norwalk, CT, USA). UV spectra were recorded on an U-2900 spectrophotometer (Hitachi, Tokyo, Japan). The analytical chiral packed column Chiralpak AD-H (250 × 4.6 mm2 ID, 5 μm) (Daicel Chemicals, Tokyo, Japan), and an Agilent 1200 series HPLC system (Agilent, Santa Clara, CA, USA) were used for the chirality analysis. 1H, 13C NMR and 2D NMR spectra were recorded on a Bruker AV-500 NMR spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany) with tetramethylsilane (δ 0.0 p.p.m.) as the internal standard. 1H NMR data were reported as follows: chemical shift (multiplicity: singlet (s), doublet (d), triplet (t) and multiplet (m)), coupling constants (Hz); 13C NMR data were reported as follows: chemical shift (quaternary carbon (C), methine (CH), methylene (CH2), methyl (CH3)). Deuterated NMR solvents were purchased from Cambridge Isotopes (Andover, MA, USA)
Isolation and taxonomic studies of the strain SCSIO 03039
A sediment sample was collected aseptically with a grab-bucket collection sampler from the location (E 87°59.7′, N 9°59.3′) at the depth of 3412 m in the Indian Ocean. The surface layer (0–10 cm) of the sediment sample was obtained as a subsample. The wet subsample was first air-dried aseptically in a laminar flow hood. Then, 2 g air-dried sample was suspended in 18 ml sterile seawater. Finally, 0.1 ml of such seawater suspensions was spread on isolation media plates containing Gauze’s medium No.1 (soluble starch 2%, KNO3 0.1%, K2HPO4 0.05%, MgSO4·7 H2O 0.05%, FeSO4·7 H2O 0.01%, pH 7.4). After incubation at 28 °C for 2 weeks, one gray white colony, designated SCSIO 03039, was isolated and deposited in the type culture collection of Center for Marine Microbiology, Research Network of Applied Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China. Isolation of genomic DNA, amplification of 16S rRNA gene by PCR, sequence comparison and phylogenetic tree construction of the strain SCSIO 03039 (Supplementary Figure S1) were performed as described previously.5
Fermentation of the strain SCSIO 03039, extraction and isolation of secondary metabolites
A single colony of SCSIO 03039 on ISP 4 medium (soluble starch 1%, K2HPO4 0.1%, MgSO4·7 H2O 0.1%, (NH4)2SO4 0.2%, CaCO3 0.2%, sea salt 3%, pH 7.0-7.4 before sterilization) was inoculated into a 250-ml Erlenmeyer flask containing 30 ml of the seed medium (soluble starch 2%, glucose 1%, bacterial protein 0.5%, yeast exact 0.5%, CaCO3 0.2%, sea salt 3%, pH 7.0 before sterilization). After incubating on a rotary shaker (200 r.p.m.) for 3 days at 28 °C, the pre-cultures were transferred to 1000-ml Erlenmeyer flask containing 200 ml of the seed medium. A total of 20 l cultures were made by this way. Fermentations were done on a rotary shaker (200 r.p.m.) for 7 days at 28 °C. The 20-l fermentation broths were extracted four times with 20 l butanone, and the mycelia cakes were extracted four times with 4 l methanol. After removing solvents, both residues from fermentation broths and mycelia cakes were combined to afford 11.86 g crude extracts.
The crude extracts were subjected to normal phase silica gel column (100–200 mesh, 90 g) and eluted with CHCl3/CH3OH (100/0, 100/1, 100/2, 100/4, 100/8, 100/16, 100/32 and 0/100, v/v, each of 360 ml) to yield eight fractions (Fr.1–Fr.8). Fr.1 and Fr.2 were combined and subjected to Sephadex LH-20 chromatography and were eluted with CHCl3/CH3OH (1:1) to give six subfractions (Fr.1-1–Fr.1-6). Fr.1-2 (23.7 mg) was further purified by semipreparative HPLC on a Varian Star Workstation, using a reversed phase column C18 (YMC*GEL ODS-A, 120 A S-5 μm, 250 × 10 mm2; solvent A, 0.1% formic acid in water; solvent B, 90% acetonitrile in water; eluted with constant 70% solvent B) to obtain compounds 7 (2.9 mg) and 8 (3.0 mg). Fr.1-5 (1.42 g) was subjected to silica gel column (100–200 mesh) and was eluted with petroleum ether/ethyl acetate (100/0, 100/10, 100/20, v/v, each of 100 ml) to give three subfractions: Fr.1-5-1 (100/0, v/v), Fr.1-5-2 (100/10, v/v) and Fr.1-5-3 (100/20, v/v). The elutions from petroleum ether/ethyl acetate (100/30, 100 ml) were collected with 10 ml each and combined on the basis of TLC analysis to give four subfractions Fr.1-5-4–Fr.1-5-7. Fr.1-5-4 (87.2 mg) was purified by semipreparative HPLC (YMC*GEL ODS-A, 120 A S-5 μm, 250 × 10 mm2; solvent A, 0.1% formic acid in water; solvent B, 90% acetonitrile in water; eluted with constant 85% solvent B) to obtain compound 1 (8.2 mg). Fr.1-5-7 (185.5 mg) was purified by semipreparative HPLC (YMC*GEL ODS-A, 120 A S-5 μm, 250 × 10 mm2; solvent A, 0.1% formic acid in water; solvent B, 90% acetonitrile in water, eluted with constant 70% solvent B) to obtain the compounds 5 (10.1 mg) and 6 (3.6 mg). Fr.3 was subjected to Sephadex LH-20 chromatography and was eluted with CHCl3/CH3OH (1:1) to give two subfractions (Fr.3-1 and Fr.3-2). Fr.3-2 (1.79 g) was separated by silica gel column chromatography (100–200 mesh) and was eluted with CHCl3/CH3OH (30:1) to give two subfractions (Fr.3-1-1 and Fr.3-1-2). Fr.3-1-2 (531 mg) was separated by normal phase silica gel column chromatography (100–200 mesh) and was eluted with petroleum ether/ethyl acetate (100/0, 100/10, 100/20, 100/30) to give four subfractions (Fr.3-1-2-1–Fr.3-1-2-4). Fr.3-1-2-2 (90.2 mg), Fr.3-1-2-3 (111 mg) and Fr.3-1-2-4 (18.1 mg) were further purified by semipreparative HPLC (YMC*GEL ODS-A, 120 A S-5 μm, 250 × 10 mm2; solvent A, 0.1% formic acid in water; solvent B, 90% acetonitrile in water) to yield compounds 2 (3.4 mg) from Fr.3-1-2-2 (eluted with constant 50% solvent B), 3 (6.7 mg) from Fr.3-1-2-3 (eluted with constant 50% solvent B) and 4 (2.6 mg) from Fr.3-1-2-4 (eluted with constant 55% solvent B), respectively.
Determining the absolute configuration of amino acids in compounds 2–4 by Marfey’s method
The FDAA derivatized hydrolysates of compounds 2 (0.50 mg), 3 (0.50 mg) and 4 (0.58 mg), and the standard amino acids were prepared according to the published method.24 The FDAA derivatives were then subjected to HPLC analysis (Luna C18 column; 5 μm, 4.6 × 150 mm2) using the following solvent system: solvent A, 10% acetonitrile in water supplementing with 0.1% formic acid; solvent B, 90% acetonitrile in water. The HPLC program for detecting FDAA derivatives of compounds 2 and 4 was set as: 5–100% solvent B (0–20 min, linear gradient), 100% solvent B (20–23 min), 100–5% solvent B (23–24 min) and 5% solvent B (24–30 min). The HPLC program for detecting FDAA derivatives of compound 3 was set as: 5–55% solvent B (0–20 min, linear gradient), 55–100% solvent B (20–24 min), 100% solvent B (24–25 min), 5–100% solvent B (25–26 min) and 5% solvent B (26–30 min). The flow rate was set at 1 ml min−1 with UV detection at λ=340 nm.
Chiral analysis of compound 2
The solution of 2 in methanol was subjected to HPLC analysis (Chiralpak AD-H, 250 × 4.6 mm2, ID 5 μm) (Daicel Chemicals) on an Agilent 1200 series HPLC system, eluted with 20% 2-propanol in n-hexane, UV detection at λ=254 nm.
Methoxyneihumicin (1): yellow needle crystal, UV/vis (in CH3OH) λmax nm (log ɛ): 202 (3.96), 227 (3.76), 310 (3.68) nm, ESI-MS m/z 357.0 [M+Na]+, 333.1 [M−H]−, HRFABMS m/z 335.1393 (calcd. for C20H19N2O3 [M+H]+, 335.1396), see Table 1 and Supplementary Figure S2 for 1H NMR and 13C NMR. X-ray crystal structure analysis of compound 1: Yellow needle crystal, C20H18N2O3, M=334.36, T=150(2) K, λ=1.54178 Å; crystal system, monoclinic, space group P 1 21/c 1, a=6.39230(10) Å, b=14.9704(3) Å, c=16.7559(3) Å, α=90°, β=91.785(2)°, γ=90°, V=1602.68(5) Å3, Z=4, Dc=1.386 Mg m−3; absorption coefficient, 0.766 mm−1, F(000)=704; and crystal size, 0.43 × 0.33 × 0.22 mm3. Theta range for data collection, 3.96–67.01°; index ranges, −7⩽h⩽5, −17⩽k⩽17, −19⩽l⩽20; reflections collected, 9663; independent reflections, 2858 (R (int)=0.0165); completeness to theta=67.01°, 99.8%; absorption correction, semi-empirical from equivalents; maximum and minimum transmission, 0.8496 and 0.7343; refinement method, full-matrix least-squares on F2; data/restraints/parameters, 2858/0/228; goodness-of-fit on F2, 1.042; NFinal R indices (I>2 sigma (I)), R1=0.0342, wR2=0.0856; R indices (all data), R1=0.0376, wR2=0.0882; and largest difference peak and hole, 0.206 and −0.230 e.Å−3. Crystallographic data (excluding structure factors) for compound 1 in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication number CCDC 888963. Copies of the data can be obtained free of charge on application to CCDC.
3-Benzylpiperazine-6-(4-methoxybenzylidene)-2,5-dione (a mixture of nocazine D (2a, 6R) and nocazine E (2b, 6S)): white powder, UV/vis (in CH3OH) λmax nm (log ɛ): 204 (3.85), 311 (3.44) nm, ESI-MS m/z 345.1 [M+Na]+, 321.3 [M−H]−, HRFABMS m/z 345.1215 (calcd. for C19H18N2O3Na [M+Na]+, 345.1215; see Table 1 and Supplementary Figure S3 for 1H and 13C NMR data.
(S, Z )-3-Benzylidene-6-methylpiperazine-2,5-dione (3): white powder, UV/vis (in CH3OH) λmax nm (log ɛ): 219 (4.52), 292 (3.75) nm, ESI-MS m/z 217.4 [M+H]+, 215.1 [M−H]−; HRFABMS m/z 215.0817 (calcd. for C12H11N2O2 [M−H]−, 215.0815); [α]21D=−239° (c=0.14, CH3OH); see Table 1 and Supplementary Figure S4 for 1H and 13C NMR data.
(S, Z )-3-Benzylidene-6-isopropylpiperazine-2,5-dione (4): white needle crystal, UV/vis (in CH3OH) λmax nm (log ɛ): 224 (3.65), 296 (3.74) nm, ESI-MS m/z 267.3 [M+Na]+, 245.2 [M+H]+, 243.2 [M−H]−, HRFABMS m/z 245.1294 (calcd. for C14H17N2O2 [M+H]+, 245.1290), 267.1115 (calcd. for C14H16N2O2Na, [M+Na]+, 267.1109); [α]21D=−2.5° (c=0.08, CH3OH); see Supplementary Figure S5 for 1H NMR data.
(3 Z , 6 Z )-3-(4-Methoxybenzylidene)-6-(2-methylpropylidene)piperazine-2,5-dione (5): white needle crystal, ESI-MS m/z 309.2 [M+Na]+, 285.3 [M−H]−; see Supplementary Figure S6 for 1H and 13C NMR data.
XR334 (6): yellow powder, ESI-MS m/z 321.1 [M+H]+, 343.1 [M+Na]+; see Supplementary Figure S7 for 1H and 13C NMR data.
2-Methoxynaphthalene-1,4-dione (7): yellow powder, ESI-MS m/z 211.2 [M+H]+, 243.2 [M+Na]+; see Supplementary Figure S8 for 1H and 13C NMR data.
1-Hydroxy-4-methoxy-2-naphthoic acid (8): brown powder, ESI-MS m/z 241.0 [M+Na]+, 217.1 [M−H]−; see Supplementary Figure S9 for 1H and 13C NMR data.
Biological activity
Antibacterial activities were evaluated against bacterial strains S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, B. subtilis SCSIO BS01 and B. thuringiensis SCSIO BT01 according to previously described methods.36 Cytotoxic activities were determined against three tumor cell lines, including MCF-7 cells (human breast adenocarcinoma cell line), NCI-H460 (human non-small cell lung cancer cell line) and SF-268 (human glioma cell line), according to published protocols.37
Accession codes
References
Goodfellow, M. & Fiedler, H. P. A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Van Leeuwenhoek 98, 119–142 (2010).
Baltz, R. H. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 8, 557–563 (2008).
Bull, A. T. & Stach, J. E. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol. 15, 491–499 (2007).
Pathom-Aree, W. et al. Diversity of actinomycetes isolated from Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles 10, 181–189 (2006).
Tian, X. P. et al. Marinactinospora thermotolerans gen. nov., sp. nov., a marine actinomycete isolated from a sediment in the northern South China Sea. Int. J. Syst. Evol. Microbiol. 59, 948–952 (2009).
Tian, X. P. et al. Streptomyces nanshensis sp. nov., isolated from the Nansha Islands in the South China Sea. Int. J. Syst. Evol. Microbiol. 59, 745–749 (2009).
Tian, X. P. et al. Pseudonocardia antitumoralis sp. nov., a new deoxynyboquinone-producing actinomycete isolated from a deep-sea sedimental sample in South China Sea. Int. J. Syst. Evol. Microbiol. (e-pub ahead of print 25 May 2012 doi:10.1099/ijs.0.037135-0).
Li, J. et al. Marininema mesophilum gen. nov., sp. nov., a thermoactinomycete isolated from deep sea sediment, and emended description of the family Thermoactinomycetaceae. Int. J. Syst. Evol. Microbiol. 62, 1383–1388 (2012).
Tian, X. P. et al. Streptomyces nanhaiensis sp. nov., a marine streptomycete isolated from a deep-sea sediment. Int. J. Syst. Evol. Microbiol. 62, 864–868 (2012).
Niu, S. et al. Lobophorins E and F, new spirotetranate antibiotics from a South China Sea-derived Streptomyces sp. SCSIO 01127. J. Antibiot 64, 711–716 (2011).
Huang, H. et al. Antimalarial β-carboline and indolactam alkaloids from Marinactinospora thermotolerans, a deep sea isolate. J. Nat. Prod. 74, 2122–2127 (2011).
Zhang, W. et al. Spiroindimicins A-D: new bisindole alkaloids from a deep-sea-derived actinomycete. Org. Lett. 14, 3364–3367 (2012).
Li, S. et al. Pseudonocardians A–C, new diazaanthraquinone derivatives from a deap-sea actinomycete Pseudonocardia sp. SCSIO 01299. Mar. Drugs 9, 1428–1439 (2011).
Huang, H. et al. Cytotoxic angucycline class glycosides from the deep sea actinomycete Streptomyces lusitanus SCSIO LR32. J. Nat. Prod. 75, 202–208 (2012).
Macherla, Y. Poster 3rd Europ. Conf. Marine Nat. Prod. (Elmau Castle, Germany, (2002).
Blake, K. & Sammes, P. Geometrical isomerism and tautomerism of 3-arylidene-6-methyl-piperazine-2, 5-diones. J. Chem. Soc. C 980–984 (1970).
Wang, L. X., Shi, Y. Z., Xu, M. H. & Hu, H. W. Condensation of 1,4-diacetyl-2,5-piperazinedione with aldehydes promoted by ultrasonically dispersed potassium. Org. Prep. Proc. Int. 28, 226–230 (1996).
Schneemann, I. et al. Nocapyrones A-D, gamma-pyrones from a Nocardiopsis strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 73, 1444–1447 (2010).
Bryans, J. et al. Inhibition of plasminogen activator inhibitor-1 activity by two diketopiperazines, XR330 and XR334 produced by Streptomyces sp. J. Antibiot 49, 1014–1021 (1996).
Little, J. E., Sproston, T. J. & Foote, M. W. Isolation and antifungal action of naturally occurring 2-methoxy-1, 4-naphthoquinone. J. Biol. Chem. 174, 335–342 (1948).
Ding, Z. S., Jiang, F. S., Chen, N. P., Lv, G. Y. & Zhu, C. G. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. Molecules 13, 220–229 (2008).
Pfefferle, C., Breinholt, J., Gurtler, H. & Fiedler, H. P. 1-Hydroxy-4-methoxy-2-naphthoic acid, a herbicidal compound produced by Streptosporangium cinnabarinum ATCC 31,213. J. Antibiot. 50, 1067–1068 (1997).
Stackebrandt, E. & Goebel, B. M. Taxonomic note: a place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44, 846–849 (1994).
Fu, P. et al. Alpha-pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10-5. J. Nat. Prod. 74, 2219–2223 (2011).
Shin, C., Kato, H., Yonezawa, Y. & Hayakawa, M. Synthesis and structural assignment of naturally-occurring 3-benzyl-6-benzylidene-2,5-piperazinedione. Heterocycles 14, 1767–1770 (1980).
Marcuccio, S. M. & Elix, J. A. Pyrazine chemistry.5. Synthesis of methylanhydropicroroccellin and dimethylpicroroccellin. Aust. J. Chem. 38, 1785–1796 (1985).
Sterns, M., Patrick, J. M., Patrick, V. A. & White, A. H. Conformational studies of some piperazine-2,5-diones—crystal-structures of 3 isomeric forms of methylanhydropicroroccellin (derivative of a lichen diketopiperazine) and a derivative bromohydrin. Aust. J. Chem. 42, 349–364 (1989).
Marfey, P. Determination of D-amino acids.2. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 49, 591–596 (1984).
Martins, M. B. & Carvalho, I. Diketopiperazines: biological activity and synthesis. Tetrahedron 63, 9923–9932 (2007).
Borthwick, A. D. 2,5-Diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 112, 3641–3716 (2012).
Schultz, A. W. et al. Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides of marine actinobacterial origin. J. Am. Chem. Soc. 130, 4507–4516 (2008).
Raju, R. et al. Naseseazines A and B: a new dimeric diketopiperazine framework from a marine-derived actinomycete, Streptomyces sp. Org. Lett. 11, 3862–3865 (2009).
Yang, L. M., Wu, R. Y., McPhail, A. T., Yokoi, T. & Lee, K. H. Neihumicin, a new cytotoxic antibiotic from Micromonospora neihuensis. II. Structural determination and total synthesis. J. Antibiot. 41, 488–493 (1988).
Raju, R., Piggott, A. M., Huang, X. C. & Capon, R. J. Nocardioazines: a novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 13, 2770–2773 (2011).
Li, B., Chen, G., Bai, J., Jing, Y. K. & Pei, Y. H. A bisamide and four diketopiperazines from a marine-derived Streptomyces sp. J. Asian. Nat. Prod. Res. 13, 1146–1150 (2011).
Xiao, Y. et al. Characterization of tiacumicin B biosynthetic gene cluster affording diversified tiacumicin analogues and revealing a tailoring dihalogenase. J. Am. Chem. Soc. 133, 1092–1105 (2011).
Wu, Z. C., Li, D. L., Chen, Y. C. & Zhang, W.M. A new isofuranonaphthalenone and benzopyrans from the endophytic fungus Nodulisporium sp. A4 from Aquilaria sinensis. Helv. Chim. Acta 93, 920–924 (2010).
Acknowledgements
This study was supported in part by the Chinese Academy of Sciences for Key Topics in Innovation Engineering (KZCX2-YW-JC202), the Ministry of Science and Technology of China (2012AA092104, 2010CB833805) and the National Science Foundation of China (41006089, 41106143, 31125001). CZ is a scholar of the ‘100 Talents Project’ of the Chinese Academy of Sciences (08SL111002). This study was funded by the Natural Science Funds of South China Sea Institute of Oceanology for Young Scholar (SQ200903). We are grateful to analytical facilities in the South China Sea Institute of Oceanology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Q., Li, S., Chen, Y. et al. New diketopiperazine derivatives from a deep-sea-derived Nocardiopsis alba SCSIO 03039. J Antibiot 66, 31–36 (2013). https://doi.org/10.1038/ja.2012.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2012.88
Keywords
This article is cited by
-
Modifications of diketopiperazines assembled by cyclodipeptide synthases with cytochrome P450 enzymes
Applied Microbiology and Biotechnology (2021)
-
Comparative studies on similarities and differences of cyclodipeptide oxidases for installation of C–C double bonds at the diketopiperazine ring
Applied Microbiology and Biotechnology (2020)
-
Non-lipopeptide fungi-derived peptide antibiotics developed since 2000
Biotechnology Letters (2019)
-
Microbial diversity of saline environments: searching for cytotoxic activities
AMB Express (2017)
-
New diketopiperazine derivatives with cytotoxicity from Nocardiopsis sp. YIM M13066
The Journal of Antibiotics (2017)