Abstract
Gombapyrones A–D, new members of the α-pyrone family of secondary metabolites, were produced by Streptomyces griseoruber Acta 3662, which was isolated from bamboo tree rhizosphere. The strain was characterized by its morphological and chemotaxonomical features and by 16S rDNA sequencing as S. griseobuber. The gombapyrone structures were determined by mass spectrometry and by NMR experiments, and were found to have an inhibitory activity against protein tyrosine phosphatase 1B and glycogen synthase kinase 3β.
Similar content being viewed by others
Introduction
In our screening program to detect novel secondary metabolites from actinomycetes for pharmaceutical applications (http://www.actapharm.org) freshly isolated strains from selected European and Malaysian ecosystems were included. The strains were grown as submerged cultures in different complex media, extracts were prepared from mycelia and from culture filtrates at various fermentation times and were screened by HPLC-diode array analysis to determine their chemical diversity. Evaluation of chromatograms was performed using our in-house developed HPLC-UV-Vis database, which contains 933 entries, most of them being antibiotics.2 Strain Acta 3662, which was isolated from soil collected from the rhizosphere of bamboo trees in the tropical rainforest of the University of Malaya Field Station at Gombak, Selangor, Malaysia, was of interest because of the presence of a family of metabolites in the mycelium extract having nearly congruent UV-Vis spectra, which were unlike those of all reference compounds of the database. This study describes the taxonomy of the producing strain, the fermentation, isolation, structural elucidation and biological activity of the new family of metabolites from strain Acta 3662, which were named gombapyrones, composed from the collection site Gombak and the α-pyrone scaffold of their structures, which are shown in Figure 1.
Results
Taxonomy of the producing strain
Strain Acta 3662 was isolated from a rhizospheric soil sample from a patch of bamboo trees at the University of Malaya Field Station in Gombak, Selangor on the West Coast of Malaysia. The putative Streptomyces strain was characterized for growth morphology on inorganic salt–starch agar (ISP 4) by electron microscopic studies. The aerial mycelium of strain Acta 3662 grown on ISP 2 agar was extensively branched and the ends of the hypha had spiral arthrospore chains. No sclerotic granules, sporangia or zoospores were observed (Figure 2). Furthermore, the strain contained L,L-diaminopimelic acid in petidoglucan. The cultural and phenotypic properties of the strain are given in Tables 1 and 2.
Strain Acta 3662 grew on a wide range of media and its cultural characteristics are given in Table 1. The color of the spore mass was gray on yeast extract–malt extract agar and on inorganic salt–starch agar. Melanoid pigment was observed when Acta 3662 was grown on peptone–yeast extract–iron agar. There was no pigment production by the strain in tyrosine agar. The carbon source utilization pattern and physiological properties are summarized in Table 2. The strain was positive for H2S formation, had strong proteolytic activity, coagulated milk, reduced nitrate to nitrite and could grow in concentrations of sodium chloride up to 6% (w/v) in media ISP 2. The carbon sources utilized are given in Table 2. Growth of strain Acta 3662 was observed from 25 to 35 °C, but the strain failed to grow at 45 °C. On the basis of growth and physiological characteristics, strain Acta 3662 was assigned to the genus Streptomyces. 16S rDNA analysis indicated that the strain is the same as Streptomyces griseoruber JCM 4642 (AY999750) with 100% similarity (Figure 3).
Screening, fermentation and isolation
Strain Acta 3662 was cultivated in 500-ml Erlenmeyer flasks in various complex media, and extracts from the mycelium and culture filtrates were prepared at 72 and 120 h, respectively, to investigate their secondary metabolite pattern by HPLC-diode array analysis. Evaluation by means of our in-house developed HPLC-UV-Vis database revealed the presence of elaiophylin and bafilomycins in the mycelium extract besides a family of metabolites of which the UV-Vis spectra differed from those of all reference compounds stored in the database. The HPLC elution profile of the mycelium extract is shown in Figure 4.
A scale-up of the cultivation of strain Acta 3662 was performed to the 10-l fermentor scale in a complex medium in which maximal biomass was reached after incubation for 120 h. Production of 1–5 started at 72 h, reaching a maximal yield of 40 mg l−1 in the case of the main component 3 after incubation for 120 h. Compounds 1–5 were isolated from mycelium by extraction with MeOH-Me2CO. Extracts were concentrated to an aqueous residue and were re-extracted with cyclohexane to separate elaiophylin, which is not soluble in cyclohexane. The crude product was purified by subsequent chromatography on a silica gel and Sephadex LH-20 column (Amersham, Freiburg, Germany). Pure gombapyrones were obtained by preparative reversed-phase HPLC as yellow powders after concentration to dryness.
Structural elucidation
The physio-chemical properties of compounds 1–5 are summarized in Table 3. The mass spectrum derived from HPLC-ESI-MS chromatograms for 1–5 showed molecular masses at [(M+H-H2O)+=389.2], [(M+H-H2O)+=389.2], [(M+H)+=377.2], [(M+H)+=377.2] and [(M+H)+=391.2], respectively. The exact molecular masses were determined by high-resolution electrospray ionization fourier transform ion cyclotron resonance MS (ESI-FT-ICR-MS) as 389.211273 Da [(M+H-H2O)+] (1), 389.211169 Da [(M+H-H2O)+] (2), 377.21054 Da [(M+H)+] (3), 377.21000 Da [(M+H)+] (4) and 391.226793 Da [(M+H)+] (5); this corresponds to the molecular formulae C26H30O4 (1) [(M+H-H2O)+theor=389.21176, Δm=0.39 p.p.m.], C26H30O4 (2) [(M+H-H2O)+theor=389.21176, Δm=0.12 p.p.m.], C25H28O3 (3) [(M+H)+theor=377.21167, Δm=1.54 p.p.m.], C25H28O3 (4) [(M+H)+theor=377.21167, Δm=0.28 p.p.m.] and C26H30O3 (5) [(M+H)+theor=391.22732, Δm=0.06 p.p.m.].
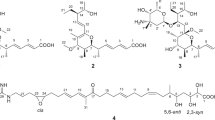
The 1H-NMR spectrum of 1 showed 10 signals in the olefinic/aromatic region, two signals between 4 and 5 p.p.m. and five signals in the aliphatic region. An integration of the signals revealed that the signal at ΔH 1.89 p.p.m. in the aliphatic region corresponded to six protons, and each of three other signals in the same region corresponded to three protons, suggesting the presence of five methyl groups in the compound. The 13C-NMR and distortionless enhancement by polarization transfer (DEPT) spectra confirmed the assumption of the presence of five methyl groups, including one methoxy group at ΔC 60.4 p.p.m. In addition, the DEPT spectrum revealed the presence of two methylene, 10 methine and nine quaternary carbons. The correlation of 1H-NMR signals to the corresponding C-atoms was carried out by the heteronuclear single quantum correlation (HSQC) NMR experiment and the signal at ΔH 1.89 p.p.m. was assigned to the corresponding 13C-chemical shifts for two methyl groups. One signal in the 1H-NMR-spectrum at ΔH 4.85 p.p.m. could not be assigned to any C-atom, suggesting the presence of a hydroxyl group. The structure of 1 was fully elucidated using COSY and heteronuclear multibond correlation (HMBC) spectra. The 1H-1H-COSY experiment showed correlations between H2-7 to H-8, H-10 to H-11, H-11 to H-12, H-12 to H-13, H-15 to H-16, H-20 to H-21, H-21 to H2-22 and H2-22 to OH-23 (Figure 5). The HMBC spectrum of 1, for which selected correlations are shown in Figure 5, provides evidence for an α-pyrone that is linked to an aromate by three conjugated double bonds. This core structure is decorated by methyl groups (C-3, C-5, C-9, C-17), one methoxy group (C-4) and a hydroxypropenyl residue (C-19), respectively. As a conclusion from the NMR-spectrometric data, compound 1 was found to be identical with the BE 51068 described previously.3 On the basis of coupling constants, the above-mentioned report has established the geometry of the double bonds at C-10/C-11 (J=15.6 Hz) as E, at C-12/C-13 (J=11.6 Hz) as Z and at C-20/C-21 (J=15.6 Hz) as E, which is identical to those determined for compound 1. These configurations have been confirmed by our groups by 1H/1H-NOESY experiments (correlations from H-8/H-10, H-10/H-15 and H-13/H-20) and, as a consequence, we could establish the E-configuration for C-8/C-9.
(a) 1H-1H COSY and HMBC correlations of BE 51068 (1); (b) selected 1H-1H COSY and HMBC correlations of gombapyrone A (2); (c) selected 1H-1H COSY and HMBC correlations of gombapyrone D (5); (d) selected 1H-1H COSY and HMBC correlations of gombapyrone B (3); (e) selected 1H-1H COSY and HMBC correlations of gombapyrone C (4).
Compound 2 has the same molecular formula as 1 (C26H30O4): however, the comparison of the 1H-NMR and 13C-NMR chemical shifts revealed minor structural differences (see Table 4). The 13C-NMR spectra with signals for C-22 at ΔC 61.6 p.p.m. (1) and at ΔC 113.3 p.p.m. (2) indicate that the latter clearly corresponds to an olefinic methylene group, whereas the methylene group in 1 is attached to an oxygen atom. In the 1H-NMR spectrum, methylene protons H2-22 of 2 appeared at ΔH 4.99/5.15 p.p.m. (d, d, J=10.3, 17.1 Hz), whereas for 1, they were assigned at ΔH 4.09 p.p.m. (t, J=5.3 Hz). Furthermore, signals of H-20/C-20 of 1 appeared at ΔH 6.65 p.p.m. (d, J=15.8) and 129.7 p.p.m. compared with ΔH 5.19 p.p.m. (t, J=4.9) and ΔC 70.1 p.p.m. for 2, which is an oxygenated methine carbon. The 1H-1H-COSY, HSQC and HMBC spectra for 2 provided proof of the deduced structure, particularly by HMBC correlations from H2-22 to C-20 and C-21, from H-20 to C-14, C-18, C-21 and C-22, and from the hydroxyl proton to C-20 and C-21 (Figure 5). As compound 2 has a stereocenter at C-20 and is therefore supposed to be optically active, the optical activity of 2 was determined as shown in Table 3.
Compound 5 with a molecular formula of C26H30O3 lacks one oxygen atom compared with 2 (C26H30O4). The corresponding 1H-NMR spectrum of 5 showed an absence of hydroxymethine signals and the appearance of one methylene signal in the aliphatic region, which suggests the replacement of the hydroxyl of 2 with a hydrogen proton in 5. Similarly, the absence of oxygen leads to a high-field shift of carbon C-20 in the 13C-NMR spectrum from ΔC 70.1 p.p.m. (2) to ΔC 37.2 p.p.m. (5), and minor signal shifts of aromatic ring carbons. 2D NMR experiments, including 1H-1H-COSY, HSQC and HMBC, confirmed the structure as shown in Figure 5.
The molecular formula determined for 3 (C25H28O3) suggests the lack of a methyl group compared with that of 5. Structural difference was located at carbon C-4, by the absence of the characteristic signals of the methoxy group in the 1H-NMR spectrum ΔH 3.78 p.p.m., (s, 3H) and the 13C-NMR spectrum ΔC 60.4 p.p.m. The expected signal for the hydroxyl group proton in the 1H-NMR spectrum of 3 was not detected. HSQC and HMBC spectra confirmed the structure of 3 (Figure 5).
Comparison of the molecular formulae of 4 (C25H28O3) and 5 (C26H30O3) suggested the lack of a methyl group in 4. In the aliphatic region of the 1H-NMR of 4, three signals assigned to three methyl carbons are found, whereas the same region of the 1H-NMR of 5 has three signals equivalent to four methyl carbons. In addition, the 1H-NMR spectrum of 4 shows a new signal at ΔH 6.21 p.p.m., (m, H). The 13C-NMR and DEPT spectra of 4 revealed the presence of three methyl, one methoxy, three methylene, three aromatic, seven methine and eight quaternary carbons. Compared with 5, signals for the methyl group (C-25) and the quaternary carbon (C-9) are replaced by an additional methine signal at ΔC 132.8 p.p.m. The HSQC, 1H-1H COSY and HMBC spectra confirmed the structure of 4 (Figure 5).
The structural element unifying compounds 2–5 is the configuration of the double bonds at C-8/C-9, C-10/C-11 and C-12/C-13. The configuration has been determined as Z for the double bonds at C-10/C-11 and C-12/C-13, on the basis of the coupling constants for H-10/H-11 (J=7.4 Hz (2), 5.6 Hz (3), 9.7 Hz (4) and 5.8 Hz (5)), and for H-12/H-13 (J=11.2 Hz (2–5)), as shown in Table 2. In addition, the geometry of the C-8/C-9 double bonds of compounds 2, 3 and 5 was assigned as E on the basis of the chemical shifts of a related system,4 and the configuration of compound 4 was assigned as E on the basis of the coupling constant for H-8/H-9 (J=15.2 Hz). The NOESY spectrum of compound 2 showed correlations for H-20 to H-13 and H-18, H-11 showed correlations to H-15 and H-10 showed correlations to H3-25. As a consequence, these correlations confirm the suggested configurations of the double bonds of compounds 2–5.
Biological activity
Except for gombapyrone A (2), no growth inhibitory activities against Gram-negative and Gram-positive bacteria or against the yeast Candida glabrata could be detected. Compound 2 inhibited the growth of Propionibacterium acnes and Staphylococcus lentus slightly with IC50 values of 100 μM.
All of the tested compounds 1–5 inhibited glycogen synthase kinase 3β (GSK-3β) with an IC50 >100 μM (inhibition at 100 μM was 23% for 1, 42% for 2, 35% for 3, 45% for 4 and 30% for 5). GSK-3 is an important regulator of glycogen synthesis and acts as a signal-transduction element, regulating several intracellular processes such as Wnt signaling.5 In addition, the β-isoform GSK-3β is supposed to be responsible for the hyperphosphorylation of the tau protein observed in pathogenesis of Alzheimer's disease.6 Only 2 showed a weak activity also at 10 μM (27% inhibition).
Gombapyrones A (2), B (3) and D (5) also exhibited a weak inhibitory activity with IC50>100 μM against human recombinant protein tyrosine phosphatase 1B (PTPN1), which is a major negative regulator of insulin signaling, by regulating the phosphorylation state of the insulin receptor and possibly insulin receptor substrate.7 Only 1 is more active compared with the others (57% inhibition at 100 μM and 23% inhibition at 50 μM), but 4 lacked activity against PTPN1. For comparison, inhibition at 100 μM was 22% for 3, 31% for 3 and 39% for 5.
DISCUSSION
Gombapyrones A–D (2–5) represent new members of secondary metabolites bearing an α-pyrone subunit as a characteristic structural feature. Although their biological activities are, in general, weak, some differences in their inhibitoring properties seemed remarkable. Antibiotic activities were found only in the case of gombapyrone A (2) and specifically against two species of bacteria. Inhibition of GSK-3β and PTPN1 was found for all gombapyrones at a high concentration level of 100 μM (except gombapyrone C, which was inactive against PTPN1). Compound BE 51068 (1) was significantly more active against PTPN1, and gombapyrone A (2) was more active against GSK-3β than were 1 and 3–5. The minor congener 1 produced by S. griseoruber Acta 3662 was found to be identical with the antitumor compound BE 51068 produced by a Streptomyces strain, which showed growth inhibition of various tumor cell lines.3
α-Pyrone metabolites are highly abundant in bacteria, fungi, plants and animals.8 They exhibit a wide range of biological activities, such as antifungal, cytotoxic, neurotoxic and phytotoxic properties, and treatment of Alzheimer's disease and high cholesterol is discussed.8, 9, 10 Furthermore, 4-hydroxy-2-pyrones have become one of the most important classes of anti-human immunodeficiency virus (HIV) agents in recent years.8 These non-peptide compounds seem to be promising candidates for the treatment of AIDS. In comparison with peptides, small α-pyrones are interesting lead candidates for synthetic strategies because of their lack of chiral centers.8, 11
Recently, we described albidopyrone, a new α-pyrone metabolite substituted in position 6 with a phenyl group, produced by a marine-derived Streptomyces strain. Albidopyrone showed an inhibitory activity against protein-tyrosine phosphatase B1, similar to gombapyrones.12 Diemenensins A and B, two polypropionate antibiotics isolated from marine pulmonate Siphonaria diemenensis, were reported to have antimicrobial activity against S. aureus and Bacillus subtilis.13 Furthermore, 11 α-pyrones, tentatively named TT-1–11, have been isolated from the imperfect fungus Trichurus terrophilus, from which compounds TT-1–4 exhibited considerably high immunosuppressive activities.14 The α-pyrone derivative, 6-[(E)-hept-1-enyl]-α-pyrone, isolated from a marine Botrytis species, exhibited a tyrosinase inhibitory activity with an IC50 value of 4.5 μM, which is more active than kojic acid showing an IC50 value of 15.5 μM, which is currently being used as a preservative in cosmetics and food.15 However, the most structurally related compounds are probably lehualides, which are isolated from a marine sponge of the genus Plakortis.16 Lehualide A is an α-pyrone with a carbon side chain resembling that of gombapyrones. Lehualides B, C and D, which also bear carbon side chains, are, however, γ-pyrones. Lehualides exhibited a broad range of biological activities; for example, lehualide A showed a mild brine shrimp toxicity (LD50 50 μg ml−1). A further structurally highly related α-pyrone metabolite from a Streptomyces strain is represented by antibiotic CRP-2504-1 from which antibacterial properties are reported.17 The new α-pyrone compounds gombapyrones A–D (2–5) extend the structural diversity of this group of natural products exhibiting enzyme-inhibitoring activities.
Methods
Producing organism and taxonomy
Strain Acta 3662 was isolated from a rhizospheric soil sample collected from a bamboo patch at the University of Malaya Field Station in Gombak, Selangor. The sample was air-dried for 3 days before dry heat treatment at 60 °C for 40 min. The heat-treated samples were then serially diluted in 0.9% (w/v) NaCl, and 0.1 ml of the 10−3 sample was plated on the surface of starch–casein agar incorporated with cycloheximide and nystatin at 50 μg ml−1 each and 20 μg ml−1 of nalidixic acid to suppress fungal and heterotrophic bacterial growth. The inoculated plates were incubated at 27 °C and observed periodically, and putative actinomycete colonies were transferred to an ISP 4 agar medium.
Cultural characteristics were determined by the methods of Shirling and Gottlieb18 and Takashi et al.19 The strain was lawned on various media such as yeast extract–malt extract agar (ISP 2), oatmeal agar (ISP 3), inorganic salts–starch agar (ISP 4), glycerol–asparagine agar (ISP 5), peptone–yeast extract–iron agar (ISP 6) and tyrosine agar (ISP 7) and incubated at 28 °C for 14–21 days. Color grouping was carried out by observing the aerial mycelium, substrate mycelium and pigmentation. The color was compared under fluorescent light and categorized on the basis of the DULUX color chart, which was used as a standard color chart.
Coverslip culture was observed for aerial and substrate mycelia and spore morphology by light and scanning electron microscope (Philips SEM 15; FEI, Singapore). Spores and spore chains on 14-day-old coverslip culture were exposed to 2% osmium tetroxide vapor for about 2 h. The coverslips were then mounted onto aluminum stubs, sputter-coated with gold and examined by s.e.m.
The hydrolysates of whole cells of strain Acta 3662 grown on ISP 2 medium were analyzed for diaminopimelic acid type.20
Carbohydrate utilization was studied by lawning the strain on ISP 2 medium (without the carbon source) and incorporated with 1% (w/v) concentrations of a range of carbon sources.18 The effect of temperature on growth was determined by streaking strain Acta 3662 on its optimum growth medium and incubating for 7 days at temperatures 15, 25, 35 and 45 °C. Tolerance against sodium chloride concentrations was determined in the same manner as for temperature studies by lawning the strain on ISP agar. Strain Acta 3662 grown on peptone–yeast extract–iron agar (ISP 6) and tyrosine agar (ISP 7) and incubated at 28 °C for 14 days was observed for melanoid formation after 7 and 14 days. Acta 3662 was grown on ISP 4 and examined for starch hydrolysis after 7 days of incubation at 28 °C. Hydrolysis of 12% (w/v) gelatin and determination of the color of the soluble pigment produced were carried out for up to 21 days of incubation. Cultures lawned on peptone–iron agar supplemented with 0.1% yeast extract were observed for hydrogen sulfide production after 6 and 18 h of incubation.
Genomic DNA extraction and polymerase chain reaction-mediated amplification of the 16S rDNA gene were carried out as described previously.21 The 16S rDNA gene sequence of the strain was aligned against 16S rRNA gene sequences of representatives of Streptomyces spp. by Basic Local Alignment Search Tool analysis.
Fermentation and isolation
Batch fermentations of strain Acta 3662 were carried out in a 10-l stirred tank fermentor (Biostat S; B. Braun, Melsungen, Germany) in a complex medium that consisted of (per liter deionized water) glucose 10 g, starch soluble 20 g, yeast extract (Ohly Kat; Deutsche Hefewerke, Hamburg, Germany) 5 g, casein peptone (Fermtech; E. Merck, Darmstadt, Germany) 5 g and CaCO3 1 g; pH was adjusted to 7.6 (5 M HCl) before sterilization. The fermentor was inoculated with 5% by volume of a shake flask culture grown in a seed medium at 27 °C in 500 ml-Erlenmeyer flasks with a single baffle for 48 h on a rotary shaker at 120 r.p.m. The seed medium consisted of glucose 10 g, glycerol 10 g, oatmeal (Neuform, Zarrentin, Germany) 5 g, soybean meal (Schoenenberger, Magstadt, Germany) 10 g, yeast extract 5 g, Bacto Casamino acids 5 g and CaCO3 1 g in 1 l tap water. Fermentation was carried out for 5 days with an aeration rate of 0.5 volume air per volume per minute and agitation at 250 r.p.m.
Hyphlo Super-cel (2%) was added to the fermentation broth and separated by multiple sheet filtration into culture filtrate and mycelium. Mycelium was extracted three times, each time with 2 l MeOH–Me2CO (1:1). The mycelium extracts were concentrated in vacuo to an aqueous residue (500 ml) that was re-extracted three times each with 350 ml cyclohexane and concentrated in vacuo to dryness. The crude extract was dissolved in CH2Cl2 and applied to a silica gel column (40 × 2.6 cm, silica gel SI 60; E. Merck). Separation was accomplished by a linear gradient from CH2Cl2 to CH2Cl2–MeOH (9:1) within 4 h at a flow rate of 5 ml min−1. Fractions containing gombapyrones (1–5) were separated on a Sephadex LH-20 column (75 × 4 cm; Amersham, Freiburg, Germany) using MeOH as eluent. To obtain pure compounds 1–5, each fraction was subjected to preparative RP-HPLC using a C-18 column (Grom-Sil 300 ODS-5 ST, 10 μm, 250 × 20 mm; Alltech Grom, Rottenburg, Germany) with CH3CN–0.1% HCOOH (gradient from 65 to 75% CH3CN over 45 min, increased to 100% CH3CN at 50 min) at a flow rate of 15 ml min−1.
HPLC-diode array analyses
The chromatographic system consisted of an HP 1090M liquid chromatograph equipped with a diode-array detector and an HP Kayak XM 600 ChemStation (Agilent, Waldbronn, Germany). Multiple wavelength monitoring was performed at 210, 230, 260, 280, 310, 360, 435 and 500 nm, and UV-Vis spectra were measured from 200 to 600 nm. A 10-ml aliquot of the fermentation broth was centrifuged, and the supernatant was adjusted to pH 5 and extracted with the same volume of EtOAc. After centrifugation, the organic layer was concentrated to dryness in vacuo and resuspended in 1 ml MeOH. Aliquots of 10 μl of samples were injected onto an HPLC column (125 × 4.6 mm) fitted with a guard column (20 × 4.6 mm) filled with 5-μm Nucleosil-100 C-18 (Maisch, Ammerbuch, Germany). The samples were analyzed by linear gradient elution using 0.1% ortho-phosphoric acid as solvent A and CH3CN as solvent B at a flow rate of 2 ml min−1. The gradient was from 0 to 100% for solvent B in 15 min with a 2-min hold at 100% for solvent B.
Structure elucidation
LC-MS experiments were performed on a QTrap 2000 (Applied Biosystems, Darmstadt, Germany) coupled to an Agilent 1100 HPLC system (Agilent). High-resolution ESI-FT-ICR mass spectra were recorded on an APEX II FTICR mass spectrometer (4.7 T, Bruker-Daltonics, Bremen, Germany) and NMR experiments were performed on a DRX 500 NMR spectrometer (Bruker, Karlsruhe, Germany) equipped with a broadband inverse detection probe head with z gradients. DMSO-d6 and CDCl3 were used as solvents for NMR experiments and chemical shifts were referenced to tetramethyl silane.
Biological activity
Antimicrobial assays were performed using B. subtilis (DSM 347), S. epidermidis (DSM 20044), S. lentus (DSM 6672), Erwinia amylovora (DSM 50901), Escherichia coli K12 (DSM 498), Pseudomonas fluorescens (NCIMB 10586), P. acnes (DSM 1897), P. aeruginosa (DSM 50071), P. syringae pv. aptata (DSM 50252), Ralstonia solanacearum (DSM 9544), Xanthomonas campestris (DSM 2405) and yeast C. glabrata (DSM 6425). The assays were prepared by transferring 50 μl of a 2 mM methanolic solution of the sample compounds into one well of a 96-well microtiter plate, evaporating the solvent in a vacuum centrifuge. Overnight cultures of the test organisms in tryptic soy broth were diluted to an OD600 of 0.02–0.06, and 200 μl of the resulting suspension was added to the wells. After incubating the microtiter plates for 15 h at 28 °C, 10 μl of a resazurin solution (0.2 mg ml−1 phosphate-buffered saline) was added to each well and the plate was incubated at 28 °C for 30 min. For evaluation of cell viability, the transformation of resazurin was assessed by measuring the intensity of fluorescence at 560Ex/590Em nm.22 The resulting values were compared with a positive (50 mg chloramphenicol for bacteria; 50 mg nystatin for yeast) and a negative control (no compound) on the same plate. P. acnes were grown anaerobically (Anaerocult A mini, E. Merck) in PYG medium (modified DSMZ-medium 104) at 37 °C for 24–48 h. The bacterial culture was diluted to an OD600 of 0.03, 200 μl of the inoculum was added to each well and the microtiter plate was incubated anaerobically at 37 °C for 48 h.
To discover specific enzyme inhibitory activities, isolated compounds were screened in several enzyme activity tests including prominent drug targets such as GSK-3β, PTPN1, phosphodiesterase 4 (PDE4), HIV-1 reverse transcriptase (HIV-1-RT) and acetylcholinesterase (AchE). Analysis of the effect on human recombinant PTPN1 was carried out with final concentrations of the substance of 10–100 μM in PTP1B assay buffer containing 100 mM Hepes buffer (pH 7.2), 2 mM EDTA, 2 mM DTT, 0.1% nonylphenylpolyethylene glycol (NP-40), 5 ng bovine serum albumin and 3 ng (150 μU) recombinant human PTP1B (Cat. no. SE332-0050, Biomol, Hamburg, Germany) in a volume of 45 μl per well. The reaction was started with 5 μl of the 1.5 mM PTP1B phosphopeptide substrate EGFR (988–998) (Cat. no. P323-0001, Biomol) dissolved in PTP1B assay buffer. After an incubation period of 15 min at 30 °C, the reaction was stopped by adding 100 μl of Biomol green (Cat. no. AK111-9090, Biomol); the ortho-phosphate concentration was quantified after incubating for 20 min at room temperature. Optimal density was measured at 620 nm using the microtiter plate reader Infinite M200 (Tecan, Crailsheim, Germany). As a positive control for inhibition of PTP1B, 50 μM of RK-682 (Cat. no. 557322–200UG, Calbiochem, Darmstadt, Germany) was added instead of the test substance.
Enzyme assays to determine GSK-3β activity were carried out as described by Baki et al.23 Measurement of AchE activity was adapted from the colorimetric assay described by Ellman et al.24 for a microplate test system. Reverse transcriptase activity was assayed using a colorimetric reverse transcriptase enzyme-linked immunosorbent assay kit (Cat. no. 11468120910, Roche, Mannheim, Germany) according to the manufacturer's instructions. Activity of PDE4 was measured by using the PDELight HTS cAMP phosphodiesterase kit (Cat. no. LT07-600, Lonza, Wuppertal, Germany) according to the manufacturer's instructions.
References
Schneider, K. et al. Lipocarbazoles, new secondary metabolites from Tsukamurella pseudospumae Acta 1857 with antioxidative activity. J. Nat. Prod. submitted (2009).
Fiedler, H.-P. Biosynthetic capacities of actinomycetes. 1. Screening for novel secondary metabolites by HPLC and UV-visible absorbance libraries. Nat. Prod. Lett. 2, 119–128 (1993).
Yamauchi, T., Nakashima, S., Hirayama, M., Ojiri, K. & Suda, K. Antitumor pyrone derivative BE 51068, its microbial manufacture, Streptomyces spp. therefore, and antitumor agents. Jpn. Kokai Tokkyo Koho (1998) 7 pp. JP 10101663, A 19980421 Heisei, AN 1998:236772.
Hengartner, U., Bernhard, K., Meyer, K., Englert, G. & Glinz, E. Synthesis, isolation, and NMR-spectroscopic characterization of fourteen (Z)-isomers of lycopene and of some acetylenic didehydro- and tetradehydrolycopenes. Helv. Chim. Acta. 75, 1848–1865 (1992).
Forde, J. E. & Dale, T. C. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 64, 1930–1944 (2007).
Mattson, M. P. Neuronal death and GSK-3beta: a tau fetish? Trends Neurosci. 24, 255–256 (2001).
Zhang, S. & Zhang, Z.-Y. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov. Today 12, 373–381 (2007).
McGlacken, G. & Fairlamb, I. J. S. 2-Pyrone natural products and mimetics: isolation, characterisation and biological activity. Nat. Prod. Rep. 22, 369–385 (2005).
Fairlamb, I. J., Marrison, L. R., Dickinson, J. M., Lu, F. J. & Schmidt, J. P. 2-Pyrones possessing promising antimicrobial and cytotoxic activities. Bioorg. Med. Chem. 12, 4285–4299 (2004).
Katritzky, A. R., Wang, Z., Wang, M., Hall, C. D. & Suzuki, K. Facile syntheses of 2,2-dimethyl-6-(2-oxoalkyl)-1,3-dioxin-4-ones and the corresponding 6-substituted 4-hydroxy-2-pyrones. J. Org. Chem. 70, 4854–4856 (2005).
Tummino, P. J., Ferguson, D., Huper, L. & Hupe, D. Competitive inhibition of HIV-1 protease by 4-hydroxy-benzopyran-2-ones and by 4-hydroxy-6-phenylpyran-2-ones. Biochem. Biophys. Res. Commun. 200, 1658–1664 (1994).
Hohmann, C. et al. Albidopyrone, a new α-pyrone-containing metabolite from marine-derived Streptomyces sp. NTK 227. J. Antibiot. 62, 75–79 (2009).
Hochlowski, J. E. & Faulkner, D. J. Antibiotics from the marine pulmonate Siphonaria Diemenensis. Tetetrahedron Lett. 24, 1917–1920 (1983).
Fujimoto, H. et al. Eleven new 2-pyrones from a fungi imperfecti, Trichurus terrophilus, found in a screening study guided by immunomodulatory activity. Chem. Pharm. Bull. 53, 923–929 (2005).
Zhang, D., Li, X., Kang, S. J., Choi, H. D. & Son, B. W. A new α-pyrone derivative, 6-[(E)-hept-1-enyl]-α-pyrone, with tyrosinase inhibitory activity from a marine isolate of the fungus Botrytis. Bull. Korean Chem. Soc. 28, 887–888 (2007).
Sata, N. et al. Lehualides A-D, metabolites from a Hawaiian sponge of the genus Plakortis,. J. Nat. Prod. 68, 1400–1403 (2005).
Doi, S. et al. Antibiotic CRP-2504-1 manufacture with Streptomyces. Jpn. Kokai Tokkyo Koho (1998) 8 pp. JP 10287666, A 19981027 Heisei, AN1998:696722.
Shirling, E. B. & Gottlieb, D. Methods for charcaterisation of Streptomyces species. Int. J. Syst. Bacteriol. 16, 313–340 (1966).
Takashi, S. et al. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. J. Antibiot. 57, 173–179 (2004).
Lechevalier, M. P. & Lechevalier, H. The chemotaxonomy of actinomycetes In Actinomycetes Taxonomy Vol. 6 (eds Dietz A. &Thayer D.W.) 227–291 (Society for Industrial Microbilogy, 1980).
Rainey, F. A., Rainey, N. W., Kroppenstedt, R. M. & Stackebrandt, E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycetes lineage: proposal of Nocardiopsaceae fam.nov. Int. J. Syst. Bacteriol. 46, 1088–1092 (1996).
Collins, L. A. & Franzblau, S. G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41, 1004–1009 (1997).
Baki, A., Bielik, A., Molnár, L., Szendrei, G. & Keserü, G. M. A high throughput luminescent assay for glycogen synthase kinase-3b inhibitors. Assay Drug Develop. Technol. 5, 75–83 (2007).
Ellman, G. L., Courtney, K. D., Andres, Jr. V. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholesterinesterase activity. Biochem. Pharmacol. 7, 88–90 (1961).
Acknowledgements
Financial support from the European Commission (project ACTAPHARM, 5th framework, QLK3-CT-2001-01783, and project ACTINOGEN, 6th framework, LSHM-CT-2004-005224) and from Bayer Schering Pharma AG (Berlin, Germany) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Article no. 52 in ‘Biosynthetic Capacities of Actinomycetes’. Article no. 51: see reference 1.
Rights and permissions
About this article
Cite this article
Helaly, S., Schneider, K., Nachtigall, J. et al. Gombapyrones, new α-pyrone metabolites produced by Streptomyces griseoruber Acta 3662. J Antibiot 62, 445–452 (2009). https://doi.org/10.1038/ja.2009.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2009.70
Keywords
This article is cited by
-
Langkocyclines: novel angucycline antibiotics from Streptomyces sp. Acta 3034
The Journal of Antibiotics (2013)
-
A strain of Streptomyces griseoruber isolated from rhizospheric soil of Chinese cabbage as antagonist to Plasmodiophora brassicae
Annals of Microbiology (2012)
-
Phenelfamycins G and H, new elfamycin-type antibiotics produced by Streptomyces albospinus Acta 3619
The Journal of Antibiotics (2011)
-
Benzoxacystol, a benzoxazine-type enzyme inhibitor from the deep-sea strain Streptomyces sp. NTK 935
The Journal of Antibiotics (2011)