Abstract
Archaea and Bacteria constitute a majority of life systems on Earth but have long been considered inferior to Eukarya in terms of solute tolerance. Whereas the most halophilic prokaryotes are known for an ability to multiply at saturated NaCl (water activity (aw) 0.755) some xerophilic fungi can germinate, usually at high-sugar concentrations, at values as low as 0.650–0.605 aw. Here, we present evidence that halophilic prokayotes can grow down to water activities of <0.755 for Halanaerobium lacusrosei (0.748), Halobacterium strain 004.1 (0.728), Halobacterium sp. NRC-1 and Halococcus morrhuae (0.717), Haloquadratum walsbyi (0.709), Halococcus salifodinae (0.693), Halobacterium noricense (0.687), Natrinema pallidum (0.681) and haloarchaeal strains GN-2 and GN-5 (0.635 aw). Furthermore, extrapolation of growth curves (prone to giving conservative estimates) indicated theoretical minima down to 0.611 aw for extreme, obligately halophilic Archaea and Bacteria. These were compared with minima for the most solute-tolerant Bacteria in high-sugar (or other non-saline) media (Mycobacterium spp., Tetragenococcus halophilus, Saccharibacter floricola, Staphylococcus aureus and so on) and eukaryotic microbes in saline (Wallemia spp., Basipetospora halophila, Dunaliella spp. and so on) and high-sugar substrates (for example, Xeromyces bisporus, Zygosaccharomyces rouxii, Aspergillus and Eurotium spp.). We also manipulated the balance of chaotropic and kosmotropic stressors for the extreme, xerophilic fungi Aspergillus penicilloides and X. bisporus and, via this approach, their established water-activity limits for mycelial growth (∼0.65) were reduced to 0.640. Furthermore, extrapolations indicated theoretical limits of 0.632 and 0.636 aw for A. penicilloides and X. bisporus, respectively. Collectively, these findings suggest that there is a common water-activity limit that is determined by physicochemical constraints for the three domains of life.
Similar content being viewed by others
Introduction
Water availability (water activity (aw)) determines both the vitality and functionality of living systems. The majority of microbes cannot multiply below 0.900 aw (Brown, 1976; Manzoni et al., 2012; Moyano et al., 2013) and for the most extremophilic species, cell division has only been observed down to ∼0.61 aw (Pitt, 1975; Williams and Hallsworth, 2009). The established water-activity window for cell division of archaeal and bacterial life (1–0.755; see Anderson, 1954; Grant, 2004) is narrower than that of some xerophilic fungi that are even able to grow and/or germinate in the range 0.755–0.605 aw (Pitt, 1975; Williams and Hallsworth, 2009). Hence the maxim that eukaryotic systems have evolved levels of solute tolerance superior to those of prokaryotes (Pitt, 1975; Brown, 1976; Williams and Hallsworth, 2009; Rummel et al., 2014).
Microbes are exposed to hostile conditions because of the spatial heterogeneity of their habitats and the temporal dynamics of environmental stress parameters (Lomstein et al., 2012; Cray et al., 2013a; Valentine, 2013; Rummel et al., 2014), as well as collateral damage induced to their macromolecular systems by the solute activities of their own chaotropic and hydrophobic metabolites (Hallsworth, 1998; Bhaganna et al., 2010; Cray et al., 2013a, b, 2014; Ball and Hallsworth, 2014). Recent studies have addressed the means by which temperature, chaotropicity, hydrophobicity, pH and radiation determine the limits of the functional biosphere (for examples see Kashefi and Lovley, 2003; Cowan and Tow, 2004; Hallsworth et al., 2007; Bhaganna et al., 2010; Chin et al., 2010; Golyshina, 2011; Cray et al., 2013b; Harrison et al., 2013; Krisko and Radman, 2013; Yakimov et al., 2014). In contrast, there is a paucity of studies to investigate whether physiological processes can occur in low water-activity environments hitherto considered hostile to biological activity. From cultivation-based studies, the majority of Archaea appear to be extremophilic, whereas Bacteria account for the majority of the biodiversity on Earth (Whitman et al., 1998). It is some members of these domains that hold current records for microbial stress tolerance towards high temperature, chaotropicity, acidity and radiation (Cowan and Tow, 2004; Baker-Austin and Dopson, 2007; Hallsworth et al., 2007; Golyshina, 2011; Cray et al., 2013a; Krisko and Radman, 2013; Yakimov et al., 2014; this article is concerned with the ability to retain metabolic activity and undergo cell division rather than the ability to survive in a dormant condition).
In consequence, we consider it highly unlikely that the cellular biology of these prokaryotes is less capable than that of stress-tolerant members of the Eukarya at high solute concentrations. This study was therefore carried out to determine whether there is a common water-activity limit for the three domains of life; we hypothesised that: (1) halophilic Archaea and Bacteria are capable of cell division below the established 0.755 water-activity limit, that is, this limit is an artefact created by the solubility limit of NaCl rather than a product of their inherent biology; and (2) the most resilient Archaea, Bacteria and Eukarya are equally tolerant to low water-activity.
Materials and methods
Organisms and media
A series of experimental, culture-based studies were carried out to determine water-activity limits for Archaea and Bacteria at high-salt concentrations (Figures 1 and 2, Table 1 and Supplementary Table S1), for Eukarya on high-sugar substrates (Figures 3, 4, 5 and Supplementary Table S1) and at high-salt concentrations (Supplementary Tables S1 and S2), and for Bacteria at high-sugar concentrations (Supplementary Tables S1 and S3). Details of microbial strains and culture media are given below; for those microbes where growth rates had been derived previously (see Supplementary Table S1; limits were determined as described in sections on ‘Water-activity measurement’ and ‘Determination of water-activity windows for biotic activity’).
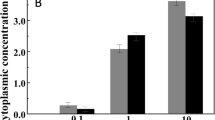
Growth in relation to water activity and/or lower water-activity values at which growth was observed for halophilic Bacteria and Archaea cultured in high-magnesium saline substrates (except for data for H. saccharovorum and Salinicola strain LC26 that were obtained in high-NaCl media (see Table 1 and Supplementary Table S1), and for S. ruber and S. longa on NaCl-supplemented media indicated by black lines in (e) and (f) respectively). (a) Halophilic Archaea Halorubrum saccharovorum (strain NCIMB 2081T; shown in brown), Halobacterium sp. NRC-1 and Halococcus morrhuae (strain NCIMB 787T; both represented in yellow), Haloquadratum walsbyi (strain DSM 16790; black), Halococcus salifodinae (strain DSM 13046; purple), Halobacterium noricense (strain DSM 15987T; hashed) and Natrinema pallidum (strain NCIMB 777T; green), and Bacteria Salinicola strain LC26 and Pontibacillus strain AS2 (both represented in grey) cultured in media supplemented with various concentrations of NaCl and MgCl2 and/or glycerol and ethylene glycol to give a range of water-activity values and incubated at 20 °C for Pontibacillus strain AS2 and Salinicola strain LC26 or 37 °C for all other species (see Table 1 and Supplementary Table S1). (b) Haloarchaeal strains GN-2 and GN-5 shown in orange and black, respectively, cultured in bittern brines supplemented with peptone at 37 °C for 6 days (calculated and replotted against water activity using data from Javor, 1984). (c) A mixed halophile community (identified using DAPI; red line) and Bacteria within this community (quantified using molecular probes; black line) by inoculating a synthetic seawater medium with supplemented NaCl using crystalliser brine and incubating at 37 °C (calculated and replotted against water activity using data from Antón et al., 2000). (d) The archaeon Halobacterium strain 004.1 in a synthetic seawater medium supplemented with NaCl, MgCl2, Na2SO4 and KCl at 37 °C (see Materials and methods and Supplementary Table S2). (e) The bacterium Salinibacter ruber strain DSM 13855T in complex media supplemented with addition of water from the Dead Sea (0.812 to 0.777 aw) and without (0.840 aw) at 35 °C (yellow line) or complex media supplemented with NaCl and incubated at 37 °C (black line; calculated and replotted against water activity using data from Sher et al., 2004 and Peña et al., 2010; see also Materials and methods and Supplementary Table S6). (f) The bacterium Salisaeta longa strain DSM 21114T in complex media supplemented with addition of water from the Dead Sea (0.926 to 0.792 aw) at 35 °C (yellow line) or complex media supplemented with NaCl (black line; Materials and methods and Supplementary Table S7). For all media, water-activity values were determined as described in the Materials and methods and at the same temperature as incubation was carried out for each set of media. Curves were extrapolated via regression analyses (dotted lines; for details see Supplementary Table S1) in order to determine the theoretical water-activity minima for growth. Pink dashed lines indicate the previously accepted water-activity limit for extremely halophilic Bacteria and Archaea (see Brown, 1990; Grant, 2004; Kminek et al., 2010).
Growth curves for halophilic Bacteria and Archaea cultured in high-NaCl substrates and plotted in relation to water activity. (a) The bacterium Halorhodospira halochloris (strain and incubation temperature not specified) cultured in a defined medium supplemented with NaCl (calculated and replotted against water activity using data from Deole et al., 2013). (b) The bacterium Halorhodospira halophila (strain DSM 244T; incubation temperature not specified) cultured in a defined medium supplemented with NaCl (calculated and replotted against water activity using data from Deole et al., 2013). (c) The bacterium Halanaerobium lacusrosei (strain DSM 10165T) cultured in a complex medium supplemented with NaCl and incubated at 37 °C (calculated and replotted against water activity using data from Cayol et al., 1995). (d) The Aacterium Actinopolyspora halophila (strain ATCC 27976T) cultured in a complex medium supplemented with NaCl, after a 14-day incubation at 37 °C (calculated and replotted against water activity using data from Yoshida et al., 1991). (e) The Archaea ‘Haloarcula californiae’ (strain DSM 8905; black line) and ‘Haloarcula sinaiiensis’ (strain DSM 8928; orange line) cultured in a complex medium supplemented with NaCl and incubated at 37 °C (calculated and replotted against water activity using data from Javor et al., 1982). (f) The archaeon Halorhabdus utahensis (strain DSM 12940T) in a defined medium supplemented with NaCl and incubated at 30 °C (calculated and re-plotted against water activity using data from Wainø et al., 2000). For all media, water activity values were determined as described in the Materials and methods and at the same temperature as incubation was carried out for each set of media. Curves were extrapolated via regression analyses (dotted lines; for details see Supplementary Table S1) in order to determine the theoretical water activity minima for growth. Pink dashed lines indicate the previously accepted water activity limit for extremely halophilic Bacteria and Archaea (see Brown, 1990; Grant, 2004; Kminek et al., 2010).
Radial extension rates for two strains of the xerophilic ascomycete Aspergillus penicillioides on solid media (MYPiA) supplemented with glycerol and other solutes over a range of concentrations, buffered at various pH values and incubated at different temperatures (Supplementary Table S5) and plotted in relation to water activity: strains JH06THH (black bars) and JH06THJ (red bars). For A. penicilliodes strain JH06THH, data relating to the following media were replotted from Williams and Hallsworth (2009): 0.647, 0.656, 0.667 and 0.670 aw. Medium composition and incubation temperatures for several treatments with common water-activity values differed (that is, 0.655i, 0.655ii, 0.702i, 0.702ii 0.714i, 0.714ii, 0.795i and 0.795ii; for details see Supplementary Table S8). The black arrow indicates the lowest water-activity at which growth of each strain was observed during an incubation period of six months. The line graph shows extrapolated growth curves plotted using data obtained on the biologically permissive media only in order to determine the theoretical extent of the water-activity windows for growth of each species; the yellow dashed line indicates the original water-activity limit for hyphal growth of the most xerophilic fungi (Pitt and Christian, 1968). For growth rate values of >0.75 mm per day, variation was ±0.10 mm per day, and for those of <0.75 mm per day, variation was ±0.040 mm per day (see Williams and Hallsworth, 2009).
Radial extension rates for three strains of the xerophilic ascomycete Xeromyces bisporus on solid media (MYPiA) supplemented with glycerol and other solutes over a range of concentrations, buffered at various pH values and incubated at different temperatures (Supplementary Table S5) and plotted in relation to water activity: strains FRR 0025 (blue bars), FRR 2347 (black bars) and FRR 3443 (pink bars). For all three strains of X. bisporus, data relating to the following media were replotted from Williams and Hallsworth (2009): 0.647, 0.653, 0.655ii, 0.656, 0.665, 0.670, 0.702ii and 0.714ii aw. Medium composition and incubation temperatures for several treatments with common water-activity values differed (that is, 0.655i, 0.655ii, 0.702i, 0.702ii, 0.714i, 0.714ii, 0.795i and 0.795ii; for details see Supplementary Table S8). The black arrow indicates the lowest water-activity at which growth of each strain was observed during an incubation period of six months. The line graph shows extrapolated growth curves plotted using data obtained on the biologically permissive media only in order to determine the theoretical extent of the water-activity windows for growth of each species; the yellow dashed line indicates the original water-activity limit for hyphal growth of the most xerophilic fungi (Pitt and Christian, 1968). For growth rate values of >4.0 mm per day, variation was ±0.20 mm per day, for those between 0.75 and 4.0 mm per day, variation was ±0.10 mm per day, and for those of <0.75 mm per day, variation was ±0.040 mm per day (see Williams and Hallsworth, 2009).
Lower water-activity limits for cell division of the most xerophilic eukaryotic microbes (upper pale-blue panel) and Bacteria and Archaea thus far identified (lower pale-grey panel) on salt-supplemented substrates (mid-blue and dark-grey bars, respectively) and sugar- or polyol-supplemented substrates (dark-blue and mid-grey bars, respectively): (i) haloarchaeal strain GN-5 (Figure 1b), (ii) haloarchaeal strain GN-2 (Figure 1b), (iii) Halorhodospira halophila (strain DSM 244T; Figure 2b), (iv) Halorhabdus utahensis (strain DSM 12940T; Figure 2f), (v) Halobacterium strain 004.1 (Figure 1d), (vi) Actinopolyspora halophila (strain ATCC 27976T; Figure 2d), (vii) Halanaerobium lacusrosei (strain DSM 10165T; Figure 2c), (viii) Halorhodospira halochloris (strain not specified; Figure 2a), (ix) Bacteria within a mixed halophile community (Figure 1c), (x) Natrinema pallidum (strain NCIMB 777T; Figure 1a and Table 1), (xi) Halobacterium noricense (strain DSM 15987T; Figure 1a and Table 1), (xii) Halcococcus salifodinae (strain DSM 13046; Figure 1a and Table 1), (xiii) ‘Haloarcula californiae’ (strain DSM 8905; Figure 2e), (xiv) Haloquadratum walsbyi (strain DSM 16790; Figure 1a and Table 1), (xv) ‘Haloarcula sinaiiensis’ (strain DSM 8928; Figure 2e), [xvi) Mycobacterium parascrofulaceum (strain LAIST_NPS017), (xvii) Mycobacterium smegmatis (strain ATCC 10143), (xviii) Tetragenococcus halophilus (strains T11 and T15), (xix) Saccharibacter floricola (strain DSM 15669T), (xx) Staphylococcus aureus (strains ATCC 6538P, NA and FM1), (xxi) Asaia bogorensis (strain JCM 10569T), (xxii) Gluconacetobacter diazotrophicus (strain DSM 5601T), (xxiii) Streptomyces albidoflavus (strain JCM 4198T), (xxiv) Staphylococcus epidermidis, (xxv) Halotalea alkalilenta (strain AW-7T), (xxvi) Streptomyces rectiviolaceus (strain JCM 9092T), (xxvii) Micromonospora grisea (strain JCM 3182), (xxviii) Sarcina sp. (strain 2b), (xxix) Lactococcus lactis (strain not specified), (xxx) Micromonospora sp. (strain JCM 3050), (see Supplementary Table S3 for (xvi) to (xxx); Stevenson and Hallsworth, 2014 for (xxiii; xxvi; xxvii; xxx)), (xxxi) Basipetospora halophila (strain FRR 2787), (xxxii) Wallemia ichthyophaga (strain EXF-994), (xxxiii) Dunaliella salina (strain UTEX 200), (xxxiv) Polypaecilum pisce (strain FRR 2733), (xxxv) Dunaliella peircei (strain UTEX 2192), (xxxvi) Aspergillus penicilliodes (strain FRR 2612), (xxxvii) germination of Wallemia sebi (strain FRR 1473), (xxxviii) Eurotium halotolerans (strain EXF-4356), (xxxix) Halocafeteria seosinensis (strain EHF34), (xl) Dunaliella parva (strain UTEX 1983), (xli) Pleurostomum flabellatum (strain CCAP 1959/1), (xlii) Hortaea werneckii (strain EXF-225), (xliii) Euplaesiobystra hypersalinica (strain CCAP 1528/1), (xliv) Wallemia muriae (strain EXF-951), (xlv) Debaryomyces hansenii (strain DSM 70590); see Supplementary Table S2 for entries (xxxi) to (xlv), (xlvi) germination of Xeromyces bisporus (strain FRR 0025) on a watchglass in a humidity-controlled atmosphere (Pitt and Christian, 1968, xlvii) Zygosaccharomyces rouxii (strain not specified) on a high-sugar medium (von Schelhorn, 1950, xlviii) germination of Aspergillus echinulatus (strain not specified) on a watchglass in a humidity-controlled atmosphere (Snow, 1949, xlix) A. penicillioides (strain JH06THJ) (Figure 3), (l) X. bisporus (strain FRR 3443) (Figure 4), (li) Eurotium amstelodami (strains FRR 2792 and FRR 0475) on media supplemented with glycerol and other solutes (Williams and Hallsworth, 2009, lii) Eurotium chevalieri strain JH06THI (Williams and Hallsworth, 2009, liii) Xerochrysium xerophilium (formerly Chyrsosporium xerophilum Pitt et al., 2013) (strain CBS 153.67T) on a medium supplemented with glucose and fructose (Leong et al., 2011, liv) Eurotium repens (strain JH06JPD) on a medium supplemented with glycerol and other solutes (Williams and Hallsworth, 2009, lv) germination and growth of Eurotium halophilicum (strain FRR 2471) on a medium supplemented with glucose and fructose (Andrews and Pitt, 1987, lvi) germination of Aspergillus penicillioides (strain not specified) in complex substrates (Pitt and Hocking, 1977); (lvii) germination of Bettsia fastidia (formerly Chrysosporium fastidium, Pitt et al., 2013) (strain FRR 77) on a watchglass in a humidity-controlled atmosphere (Pitt and Christian, 1968, lviii) germination of W. sebi (strain FRR 1473) on a medium supplemented with glucose and fructose (Pitt and Hocking, 1977, lix) hyphal growth of B. fastidia (strain FRR 77) (Pitt and Christian, 1968; Williams and Hallsworth, 2009, lx) germination of Eurotium rubrum (strain FRR 0326) (Gock et al., 2003). For each bar, the shaded region extends to the lowest empirically determined water-activity value (see also Supplementary Tables S1–S3 and Figures 1, 2, 3, 4). Only lower water-activity limits for growth are indicated (unless spore germination is indicated); note that some of these species may be unable to grow close to a water activity of 1 (for examples, see Figure 1). The pink dashed line indicates the previously accepted water-activity limit for growth of the most halophilic Bacteria and Archaea (see Brown, 1990; Grant, 2004; Kminek et al., 2010); the yellow dashed line indicates the original water-activity limit for hyphal growth of the most xerophilic fungi (Pitt and Christian, 1968).
An initial assessment of water-activity limits for halophilic species of Archaea and Bacteria was carried out on saline media ranging from 0.803 to 0.642 aw (Figure 1a and Supplementary Table S4) using Halobacterium noricense (DSM 15987T), Halobacterium sp. NRC-1 (i.e. Halobacterium salinarum ATCC 700922), Halococcus morrhuae (NCIMB 787T), Halococcus salifodinae (DSM 13046), Halorubrum saccharovorum (NCIMB 2081T) and Natrinema pallidum (NCIMB 777T). These cultures were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany; Hbt. noricense and Hcc. salifodinae) and the National Collection of Industrial, Food and Marine Bacteria (NCIMB, Aberdeen, UK; Hcc. morrhuae and Hrr. saccharovorum). Hbt. sp. NRC-1 was provided by Helga Stan-Lotter (University of Salzburg, Austria). The composition for the salt-supplemented culture media used to assay these halophiles are detailed in Supplementary Table S4. Cultures were maintained in their respective media and incubated during these experiments at 37 °C. Data obtained were compared with those for Haloquadratum walsbyi (DSM 16790), Pontibacillus (strain AS2), Salinicola (strain LC26), haloarchaeal strains GN-2 and GN-5, Halorhodospira halochloris, Halorhodospira halophila strain (DSM 244T), Halanaerobium lacusrosei (strain DSM 10165T), Actinopolyspora halophila (strain ATCC 27976T), ‘Haloarcula californiae’ (strain DSM 8905), ‘Haloarcula sinaiiensis’ (strain DSM 8928) and Halorhabdus utahensis (strain DSM 12940T); see Supplementary Materials and Methods).
Studies of halophilic Archaea and Bacteria capable of growth in MgCl2-rich and/or NaCl-rich media (Figures 1b–f and 2) were carried out using pure cultures of the following strains: Halobacterium strain 004.1 (for source see Norton et al., 1993), Salinibacter ruber strain DSM 13855T and Salisaeta longa strain DSM 21114T. Cultures of Halobacterium strain 004.1 were maintained in the nutrient media on a rotary shaker at 37 °C (for details see Supplementary Table S5; Norton et al., 1993). For studies of MgCl2 tolerance, S. ruber was maintained on Modified Yeast Extract, Salts Broth (0.840 aw) containing (per litre): 1.0 g yeast extract (Beckton, Dickinson and Company, Sparks, MD, USA), 5.0 g KCl, 1.25 g CaCl2.2H2O, 0.625 g NaBr, 0.25 g NaHCO3 plus NaCl (3.34 M), MgSO4 (0.101 M) and MgCl2 (0.0801 M), and incubated at 35 °C. For studies of both NaCl and MgCl2 tolerance, S. longa was maintained in (per litre) 2.0 g soluble starch (BDH, Leicester, UK), 1.0 g yeast extract (Difco, Oxford, UK), 1.0 g casamino acids, 5.0 g K2SO4, 0.1 g CaCl2.2H2O plus NaCl (1.71 M) and MgCl2 (0.246 M), and incubated at 35 °C. The composition and water-activity values for culture media used to assay S. ruber and S. longa are detailed in Supplementary Tables S6 and S7, respectively.
Strains of Aspergillus penicillioides (JH06THH and JH06THJ) were isolated previously (Williams and Hallsworth, 2009), and are available from the corresponding author, and strains of Xeromyces bisporus (FRR 0025, FRR 2347 and FRR 3443) were obtained from CSIRO Food and Nutrition (FRR collection, North Ryde, NSW, Australia). Cultures were maintained on Malt-Extract, Yeast-Extract Phosphate Agar (MYPiA (per litre): 10 g malt-extract w/v (Oxoid, Oxford, UK), 10 g yeast-extract w/v (Oxoid), 15 g agar w/v (Acros, Bridgewater, NJ, USA), 1.0 g K2HPO4 w/v)) supplemented with either 6.52 M glycerol (A. penicillioides) or 2.2 M sucrose (X. bisporus) and incubated in sealed polyethylene bags at 30 °C. The composition and water-activity values for culture media used in stress-tolerance assays of xerophile strains are detailed in Supplementary Table S8.
Debaryomyces hansenii strain DSM 70590 was used to determine the lower water-activity limit in high-salt media (Supplementary Table S2). Cultures were maintained in Modified Yeast Extract, Casamino Acid, Tri-Sodium Citrate Broth that contained additional NaCl (3.5 M) and MgCl2 (0.399 M); 0.803 aw (see Supplementary Table S4) and incubated at 37 °C.
Quantitation of growth rates
Exponential-phase cells of Hbt. noricense, Hbt. sp. NRC-1, Hcc. morrhuae, Hcc. salifodinae, Hrr. saccharovorum, Nnm. pallidum, Pontibacillus strain AS2 and Salinicola strain LC26 were used to inoculate modified Payne’s media (250 ml in side-arm flasks; Payne et al., 1960) of varying water activities (see Supplementary Table S4) at a starting cell density equivalent to 0.2 OD600 nm. Cultures were incubated at 37 °C and cell density was determined by turbidometric estimation using a nephelometer over a period of two months (two replicates; see Table 1 and Figure 1a). Turbidometric readings were used to construct growth curves from which doubling rates (in days) during exponential growth phase were calculated (Table 1 and Figure 1a).
Exponential-phase cells of Halobacterium strain 004.1 (1.6 ml of a preculture, OD560 nm=0.5) were used to inoculate media (160 in 250 ml Erlenmeyer flasks) with salt concentrations at increasing multiples of the control medium (see Figure 1d and Supplementary Table S5). Flasks were incubated on a rotary shaker (37 °C; 225 r.p.m.) and cell density monitored turbidimetrically (OD560 nm). Cell concentration was determined with a Helber haemocytometer (Hawksley, Sussex, UK) and plotted against the water activity (see below).
Exponential-phase cells of S. ruber were used to inoculate complex media (100 in 250 ml Erlenmeyer flasks) supplemented with water from the Dead Sea, Israel (35% w/v total salts; see Figure 1e and Supplementary Table S6) to determine tolerance to MgCl2/NaCl mixtures in relation to water activity. Cultures (three replicates) were incubated at 35 °C over 6 days during which OD600 nm was regularly determined. Mean values (OD units per day) derived from exponential-phase measurements were plotted against media water-activity (Figure 1e). Exponential-phase cells of S. longa were used to determine tolerance to MgCl2/NaCl mixtures (100 in 250 ml Erlenmeyer flasks) in relation to water activity (Supplementary Table S7).
For the xerophilic fungi assayed, agar plugs (4-mm diameter) were taken from the periphery of actively growing cultures of A. penicillioides and X. bisporus and inoculated onto MYPiA supplemented with a range of stressors (Supplementary Table S8). Petri plates were sealed using Parafilm (Neenah, WI, USA) and plates of the same water activity were incubated in bags of polyethylene that allows gaseous exchange but minimises water loss (Ivanov, 2001; Huang et al., 2002; Hallsworth et al., 2003a, 2003b). Colony diameter was measured daily (in perpendicular directions) and used to calculate rates for radial hyphal extension in mm per day (see Williams and Hallsworth, 2009).
Growth of Debaryomyces hansenii (Supplementary Table S2), cultured in Modified Yeast Extract, Casamino Acid, Tri-Sodium Citrate Broth supplemented with NaCl and MgCl2 (see Supplementary Table S4), was assessed by via nephelometer (see above) for a period of two months at 37 °C. Turbidometric readings were used to construct growth curves, and doubling times during exponential-growth phase were calculated in order to determine the lower water-activity limit (Supplementary Table S2). For details relating to utilisation of growth-related data from previous studies (Supplementary Table S1), see Supplementary Materials and Methods.
Water-activity measurement
Water-activity values of media were measured using a Novasina Humidat IC-II water-activity machine (Axair Ltd, Pfäffikon, Switzerland) at the same temperature at which the relevant microbial culture was incubated. The apparatus, fitted with an alcohol-resistant humidity sensor and eVALC alcohol filter that prevents interference by volatiles such as ethanol and glycerol (Hallsworth and Nomura, 1999; Axair Ltd), was calibrated using saturated salt solutions of known water-activity (Winston and Bates, 1960). Water-activity determinations were carried out three times using replicate solutions made up on separate occasions; variation between replicate values was within ±0.002 for determinations in the range 1 to 0.900 aw, and within ±0.001 for those within the range 0.900 to 0.600 aw (see below).
Media used for stress-tolerance assays of Pontibacillus strain AS2, Salinicola strain LC26 and Hqr. walsbyi (Table 1 and Supplementary Table S1; Bolhuis et al., 2004; Sass et al., 2008), the halophilic bacterial community (largely Salinibacter) from crystalliser pond CR-30 (Braç del Port, Alicante, Spain) (Supplementary Table S6 and Figure 1b; Antón et al., 2000) and S. ruber (Supplementary Table S6 and Figure 1f; Sher et al., 2004; Peña et al., 2010) were remade for the current study, and then water-activity determinations carried out as described above. The ionic composition of brines used to culture haloarchaeal strains GN-2 and GN-5 (which was determined to an accuracy level within the low-mM range; Javor, 1984) was used to calculate the water activity of these media (Figure 1a). The values obtained were consistent with the conversion of their brine density (Bé°) to water activity by Javor (1989).
Lower water-activity limits for cell division in some of the most halophilic eukaryotes were determined by plotting extant data sets for cell division at high-salt concentrations in relation to water activity (Supplementary Tables S1 and S2). For culture media of algal species Dunaliella parva (UTEX 1983), Dunaliella peircei (UTEX 2192) and Dunaliella salina (UTEX 200), and the fungi Eurotium halotolerans (MZKI A-560), Wallemia ichthyophaga (EXF-994), Wallemia sebi (FRR 1473) and Wallemia muriae (EXF-951; Cifuentes et al., 2001; Butinar et al., 2005; Zalar et al., 2005), the water-activity values that correspond to the limit for cell division were calculated according to the concentrations of NaCl and other components of their respective culture media (Supplementary Table S2) by reference to standard water-activity curves that had been plotted using data points derived at the relevant temperature. Lower water-activity limits for cell division of the nanoflagellate species Euplaesiobystra hypersalinica (CCAP 1528/1), Halocafeteria seosinensis (EHF34) and Pleurostomum flabellatum (CCAP 1959/1) were determined by recreating their respective culture media (see Park et al., 2006, 2007, 2009), followed by empirical determinations of water activity (see below) at their respective incubation temperatures (see Supplementary Table S2).
Water-activity limits for cell division in some of the most xerotolerant Bacteria were determined by plotting extant data sets for cell division at high concentrations of sugar (or other non-ionic solutes) in relation to water activity (Supplementary Tables S1 and S3). Water activity values that corresponded to the cell-division limits for Tetragenococcus halophilus (strains T11 and T15) were determined by empirical measurements of water activity (see below) of sucrose solutions (30 °C) that were made up based on the °Bx values from Justé et al. (2008a, 2008b). Water-activity limits for Asaia bogorensis (JCM 10569T), Gluconacetobacter diazotrophicus (DSM 5601T), Saccharibacter floricola (DSM 15669T), Pseudomonas putida (DSM 6125h), Halotalea alkalilenta (AW-7T) and Rosenbergiella nectarea (8N4, LMG 26121, DSM 24150) were determined empirically (see below) using sugar solutions that were remade, and then measured at their appropriate incubation temperatures (Jojima et al., 2004; Ntougias et al., 2007; Cray et al., 2013a; Halpern et al., 2013; Supplementary Table S3).
There are various theoretical and empirical ways in which to determine water-activity values (see, for example, Winston and Bates, 1960; Norrish, 1966; Greenspan, 1977; Ferro Fontán and Chirife, 1981, Brown, 1990; Hallsworth and Nomura, 1999, Caurie, 2005; Yu et al., 2009). For undefined media and/or culture media that have been sterilised, poured into Petri plates and/or stored, water activity cannot be predicted due, in part, to water loss as vapour and hence must be determined empirically. Water-activity values obtained via any available technologies are associated with some degree of variation because of the respective limits of uncertainty and accuracy of the latter (see Yu et al., 2009). Documented water-activity values for saturated salt solutions that can be used for the calibration of instruments are relatively accurate (see Winston and Bates, 1960; Yu et al., 2009). For example, Yu et al. (2009) demonstrated overall uncertainties for water-activity measurements of only ±0.0012 to ±0.0018, and accuracy levels of 0.0002 to 0.0005. Some of the new generation of commercially available instruments, such as the Novasina Labmaster series (Axair Ltd), are limited by software that can only be calibrated at a small number of fixed water-activity values and temperature points. The Novasina Labmaster series have been programmed using theoretical water-activity values that were derived using data pooled from a small number of empirical measurements and/or a series of (untested) assumptions (Greenspan, 1977), rather than those collated from a large number of (methodologically diverse) sources—and therefore more accurate—by Winston and Bates (1960).
We took precautions to minimise potential errors that can arise from sampling protocol, instrument resolution and discrimination threshold, inaccuracy of measuring equipment and values for constants or other parameters obtained from external sources and so on (including standard values of saturated salt solutions used for calibration; Brown, 1990; Hallsworth and Nomura, 1999; Yu et al., 2009). An earlier model of water-activity apparatus was used—the Novasina Humidat IC-II (see above)—that allows for manual calibration along continuous scales of water activity and temperature, as used in our earlier studies (see, for example, Hallsworth and Magan 1994a, 1994b, 1994c, 1995, 1996; Kashangura et al., 2006; Hallsworth et al., 2007; Chin et al., 2010; Stevenson and Hallsworth, 2014; Yakimov et al., 2014); filters were fitted to prevent interference from volatiles or particulates, and these were installed taking care to prevent tearing (as described previously, Hallsworth and Nomura, 1999); saturated salt solutions were made up using highest-grade salts and these (along with the medium sample which was contained in a closed sample cup, and the Novasina Humidat IC-II sensor) were left to equilibrate at the temperature at which water-activity determinations were carried out for two to three weeks as described by Winston and Bates (1960); determinations were carried out in a Binder MK53 Environmental Simulation Chamber (Binder GmbH, Tuttlingen, Germany) to minimise temperature variation; measurements were invariably made when the sensor reading was below the water activity of the sample to avoid making the sensor desaturate during a reading; for solid media, Petri plates were poured at a temperature of only 5–7 °C above the medium gel-point (which can differ by up to 10 or 15 °C depending on stressor type and concentration; see Cray et al., 2013b) and lids were immediately placed onto plates to minimise loss of water vapour, avoid condensation and minimise any potential difference between water lost upon pouring between the first and last plates of each batch (for the same reason, agar-containing media were made in small volumes where possible and poured rapidly as practically possible); water-activity readings were taken frequently throughout each sample equilibration period in order to enable the construction of curves that help to establish levels for accuracy of values, consistency and sensor functionality; the Novasina Humidat IC-II sensor was allowed to desaturate immediately after each water-activity measurement to avoid growth of xerophilic fungi on the sensor or sensor housing; and other precautions that are listed in the following section. Uncertainty analyses for water-activity determinations using Aqualab equipment (which is based on dew-point measurements rather than humidity readings) that were carried out by Yu et al. (2009) established that equipment errors were less than those stated by the manufacturer (that is, ±0.003). This is consistent with our finding that the variation of our values using the now-obsolete Novasin Humidat IC-II (±0.001 in the range 0.900 to 0.600 aw) was less than that claimed by the manufacturer for current Labmaster models that use the same sensor technology (that is, ±0.003).
Determination of water-activity windows for biotic activity
Assessments of microbial growth rates were made on media over a range of water-activity values in order to facilitate the construction of curves to determine water-activity windows (Supplementary Table S1 and Figures 1, 2, 3; von Schelhorn, 1950; Hallsworth and Magan 1994a, 1994b; Hallsworth et al., 1998; Williams and Hallsworth, 2009). As a precaution, water-activity values of media were checked post-incubation (none of these differed from preinoculation values by more than ±0.002). Growth curves were extrapolated where necessary (Supplementary Tables S1–S3 and Figures 1, 2, 3, 4) to predict the failure point of the window. This has been carried out for data obtained from (1) planktonic cultures of diverse microbes in liquid media (von Schelhorn, 1950; Tienungoon et al., 2000; Ferrer et al., 2003; Cray et al., 2013b); (2) spot-test assays for filamentous Bacteria on solid media (Stevenson and Hallsworth, 2014); and (3) radial extension of mycelia for filamentous fungi on solid media (Hallsworth and Magan, 1999; Rosso and Robinson, 2001; Tassou et al., 2007; Williams and Hallsworth, 2009; Huchet et al., 2013). Some of these studies (Rosso and Robinson, 2001; Tassou et al., 2007; Huchet et al., 2013) demonstrated/validated theoretical determinations for water-activity minima with an accuracy consistent with the level of sensitivity of the microbial cell; that is, ±0.002 (Stevenson et al., 2014). However, where growth curves continue in an asymptotic manner, extrapolation may give a conservative estimate for water-activity minima for both fungi (see Figures 1b, 3 and 4) and planktonic cultures of prokaryotic cells (Neumeyer et al., 1997). For studies of temperature, modelling of microbial growth curves can also result in conservative predictions of the actual limits (Tienungoon et al., 2000). In the current study, linear, polynomial or Gausian regression analyses were carried out to predict water-activity minima; for each data set, the regression analysis that gave the highest regression coefficient was employed (Supplementary Table S1). In order to compare water-activity limits for the most resilient microbes known, we selected the 30 eukaryotic microbes (see Supplementary Tables S1 and S2 and Figures 3 and 4) and 30 Archaea or Bacteria (see Supplementary Tables S1 and S3 and Figures 1 and 2) with the highest recorded tolerance to ionic or non-ionic solutes (Figure 5; Stevenson and Hallsworth, 2014; Stevenson et al., 2014). These data were compiled to determine only the lower water-activity limits of microbial growth windows, and were based on biotic activity including germination rates and growth rates, as there are no data sets derived using culture-independent techniques that reveal metabolic activity at such low water-activity values to our knowledge.
Results and discussion
A water-activity limit of ∼0.611 for growth of archaeal and bacterial halophiles
A number of halophilic Archaea and Bacteria are not only capable of cell division in salt-saturated substrates, but can have substantial growth rates under these conditions (Javor, 1984; Brown, 1990; Yoshida et al., 1991; Cayol et al., 1995; Grant, 2004; Deole et al., 2013). NaCl is soluble to ∼5.2 M, which is equivalent to 0.755 aw at temperatures in the range of 25–35 °C (the water activity of saturated solutions of NaCl ranges from 0.765 to 0.745 between 2 °C and 50 °C; Winston and Bates, 1960). We selected nine extremely halophilic species of Archaea and Bacteria that belong to a range of phyla, and determined whether they would grow in liquid media supplemented with various stressors to obtain a range of water-activity values from 0.803 to 0.642 (Figure 1a, Table 1 and Supplementary Table S4). At low water-activity values, cell-replication times for the vast majority of halophiles become exponentially long (Brown, 1990). Remarkably, however, the majority of the empirically determined doubling times for these microbes ranged from <7 to ∼21 days at water-activity values of ≤0.717, and four of the selected species grew at even lower water-activity values: 0.709 for Hqr. walsbyi, 0.693 for Hcc. salifodinae, 0.687 for Hbt. noricense and 0.681 for Nnm. pallidum (Figure 1a and Table 1). The relatively high growth rates of Hqr. walsbyi, Hbt. noricense and Nnm. pallidum, despite this low water-activity range (0.709 to 0.681), suggest that their actual water-activity minima are <0.681 (likely in the range 0.650 to 0.605; Figure 1a). For some of the media assayed, the NaCl concentrations were relatively low (between 3.3 and 1.5 M) and MgCl2 concentrations were relatively high; two of the media contained ethylene glycol or glycerol (Figure 1a, Table 1 and Supplementary Table S4). When compared on a w/v basis, MgCl2 depresses water activity more effectively than NaCl (Winston and Bates, 1960; Hallsworth et al., 2007); it is, nevertheless, the potent chaotropicity-mediated stress induced by MgCl2 that typically limits microbial systems which are exposed to this salt (Hallsworth et al., 2007; Bhaganna et al., 2010; Cray et al., 2013b; Yakimov et al., 2014). It is for this reason that we substituted MgCl2 with ethylene glycol and glycerol for some treatments. Glycerol can protect against chaotrope-induced stresses in phylogenetically diverse microbes (Hallsworth et al., 2003a, 2007; Williams and Hallsworth, 2009; Bhaganna et al., 2010; Bell et al., 2013). This polyol can, however, itself act as a chaotropic stressor at molar concentrations (Williams and Hallsworth, 2009; Cray et al., 2013a). For Hbt. noricense and Hcc. salifodinae, the partial substitution of salts by either ethylene glycol or glycerol facilitated growth at lower water-activity values than occurred on media with only salts added (Table 1 and Supplementary Table S4). Given the ability of MgCl2 to reduce water activity to <0.755 (several studies suggest that MgCl2 concentrations in the range 2–3 M can be biologically permissive for some halophiles (Hallsworth et al., 2007; Oren, 2013; Yakimov et al., 2014)), we sought evidence that halophilic Archaea and Bacteria can be active at water activities <0.681 in magnesium-rich media or substrates including bitterns, that is, crystalliser ponds that have naturally elevated magnesium concentrations (Figures 1b–f).
Two strains of haloarchaea that originated from Mexican solar salterns (GN-2 and GN-5) were previously cultured in bittern water (Javor, 1984). We determined the water activity of this substrate (see Materials and methods) and plotted growth data for these strains against water activity (Figure 1b), indicating that although optimal growth was observed between 0.800 and 0.845 aw, growth rates were remarkably high at 0.755 aw; that is, 61% and 67% of that of the optimum for GN-2 and GN-5, respectively. Despite the short incubation time (6 days), both strains were active even in the most high-salt medium (equivalent to a brine density of 32 Bé°; Javor, 1984) at a water activity of 0.635 (Figure 1b). In common with other studies of water-activity and temperature windows for microbial growth (or those for other stress parameters; see Materials and methods), we carried out regression analyses to obtain theoretical water-activity minima for strains GN-2 and GN-5; these were 0.615 and 0.611, respectively (Supplementary Table S1 and Figure 1b). This represents a substantial increase in relation to the previously accepted water-activity window for cell division of archaeal or bacterial halophiles.
We also sought evidence of microbial activity at sub-0.755 water-activity values for halophilic Bacteria that are known to inhabit bittern brines. Water from a crystalliser pond (Pond CR-30, Braç del Port) was previously used to inoculate a synthetic seawater medium supplemented with NaCl, which was then monitored over time by quantifying total cell counts and using molecular probes to identify halophilic Bacteria belonging to the Salinibacter assemblage within the community (Antón et al., 2000; Figure 1c). We determined the water activity of these media (see Materials and methods) and plotted these values against growth-rate data for this Bacterial assemblage (Figure 1c). Growth rates were optimal at 0.841 aw (doubling time=12 h) and reduced by only 50% at 0.755 aw. Extrapolation of this curve suggests an actual water activity limit in the range 0.675–0.670 (Supplementary Table S1 and Figure 1c). These values were derived from a combined data set representing a number of bacterial populations, and hence it is likely that one or more populations and species is/are capable of growth at lower water activities than this extrapolation suggests.
The biotic windows were also determined for individual Archaea and Bacteria found in magnesium-rich habitats and/or known to have a degree of tolerance towards MgCl2 (strains of Halobacterium, Salinibacter and Salisaeta; Figures 1d–f). Halobacterium strain 004.1, isolated from a brine pool within a subsurface salt deposit (UK, Norton et al., 1993), shares traits in common with strains of Hbt. noricense (McGenity et al. 2000), a species that is frequently found in crystals of buried halite (Gramain et al., 2011). This strain remained highly active at 0.728 aw (∼70% of the optimum growth rate) and extrapolation of the curve suggested a lower theoretical limit of 0.658 aw (Supplementary Table S1 and Figure 1d). S. ruber can form up to 27% of halophile communities in high-magnesium habitats such as crystalliser ponds in solar salterns (Antón et al., 2000). This bacterium has extraordinary adaptations that enable it to dominate high-salt habitats, including light-activated protein pumps that generate proton-motive force and thereby boost energy generation, use of ions—rather than organic compounds—for osmotic adjustment and cytosolic proteins with physicochemical properties that enable function at high ionic strength (Oren et al., 2002; Balashov et al., 2005; Cray et al., 2013a; Oren and Hallsworth, 2014). S. longa, a close relative of Salinibacter spp. (Bodaker et al., 2010; Oren, 2013), is also found in hypersaline marine habitats (Vaisman and Oren, 2009). However, we found that high-magnesium media (supplemented with water from the Dead Sea; Supplementary Tables S6 and S7) were not biologically permissive for S. ruber and S. longa in as much as their growth windows did not extend below 0.755 (Figures 1e and f).
Given that the chaotropicity of MgCl2 may have curtailed the growth of S. ruber and S. longa (Figures 1e and f), we also determined growth rates for these strains in high-NaCl media (Figure 2). Bacteria notorious for obligate extreme halophilicity at high NaCl concentrations have been studied previously: Actinopolyspora halophila, Halanaerobium lacusrosei (formerly Haloanaerobium lacusroseus), Halorhodospira halochloris (formerly Ectothiorhodospira halochloris) and Halorhodospira halophila (formerly Ectothiorhodospira halophila; Yoshida et al., 1991; Ollivier et al., 1994; Ruan et al., 1994; Cayol et al., 1995; Imhoff and Süling, 1996; Oren, 2000; Deole et al., 2013), as well as the exceptionally halophilic Archaea ‘Haloarcula californiae’, ‘Haloarcula sinaiiensis’ and Halorhabdus utahensis (Javor et al., 1982; Wainø et al., 2000). We therefore quantified the water-activity limits for each species based on previous empirical growth determinations (Figure 2 and Supplementary Table S1). Two of these species, A. halophila and Hlr. halochloris, exhibited exceptionally high biomass/growth rates at 0.757 and 0.774 aw, respectively, in salt-saturated media that were ∼95% of those corresponding to their water-activity optima (Figures 2b and d, respectively). Unlike S. ruber, A. halophila utilizes organic compounds for osmotic adjustment and is known to accumulate high levels of compatible solutes such as ectoine and trehalose (Kar et al., 2014). Extrapolations of growth curves (Supplementary Table S1) indicated theoretical limits of 0.747 for S. longa (Figure 1f), 0.725 for S. ruber (Figure 1e), 0.710 for ‘Har. sinaiiensis’ (Figure 2e), 0.704 for ‘Har. californiae’ (Figure 2e), 0.680 for Hlr. halochloris (Figure 2a), 0.668 for H. lacusrosei (Figure 2c), 0.660 for A. halophila (Figure 2d), 0.647 for Hrd. utahensis (Figure 2f) and 0.623 for Hlr. halophila (Figure 2b). The data presented in Figures 1 and 2 are consistent with the high growth rates of other archaeal and bacterial species known to be extreme obligate halophiles (for example, Actinopolyspora iraqiensis (syn. Saccharomonospora halophila) strain IQ H2, Ruan et al., 1994; Tang et al., 2011). Furthermore, the majority of the halophilic prokaryotes represented in Figures 1 and 2 achieved optimum growth rates in the range 0.845 to 0.765 aw. This water-activity range is lower than, or equivalent to, that of the most xerophilic fungi thus far reported (Pitt, 1975; Brown, 1990; Williams and Hallsworth, 2009), with the exception of Xeromyces bisporus (see below).
Revised lower limit of 0.632 aw for hyphal growth of fungal xerophiles
Before our recent studies involving manipulation of the chao-/kosmotropicity of culture media (Williams and Hallsworth, 2009), the established water-activity limit for mycelial growth in xerophilic fungi was 0.656 (for X. bisporus, Pitt and Christian, 1968). In the current article, we attempted to extend biotic windows of xerophilic fungi via manipulations of medium composition and other environmental conditions, and in this way to emulate the study of halophilic prokaryotes described above. Determinations were made for water-activity windows for hyphal extension (rather than germination) to enable comparisons with the stress tolerance of the Archaea and Bacteria (Table 1 and Figures 1 and 2). Furthermore, vegetative growth of mycelia is qualitatively distinct from spore germination in terms of cellular ultrastructure and physiology, growth kinetics and water-activity limits (Ayerst, 1969; Pirt, 1975; Hallsworth and Magan, 1994a, 1995; Leong et al., 2011). Whereas fungal propagules are renowned for their inherent robustness (for example, during desiccation and at extreme pressures and temperatures), this tenacity is only observed during dormancy (Potts, 1994; Chin et al., 2010; Wyatt et al., 2014). For this study, we selected five strains of extremely xerophilic fungi: X. bisporus FRR 0025 (reported to grow down to 0.656 and germination down to 0.605 aw; Pitt and Christian, 1968); X. bisporus strains FRR 2347 and FRR 3443 that are capable of hyphal growth at water activities comparable to strain FRR 0025 (Williams and Hallsworth, 2009); and Aspergillus penicilliodes strains JH06THH and JH06THJ that have been reported to be more xerophilic than X. bisporus (the former were capable of mycelial growth down to 0.647 aw; Williams and Hallsworth, 2009).
The water activity for optimum growth of A. penicillioides was found to be between 0.800 and 0.820 (Figure 3); lower than that for X. bisporus, other species of xerophilic fungi (Figure 4; Williams and Hallsworth, 2009) and most halophilic Archaea and Bacteria (Figures 1 and 2). Radial growth rates for A. penicillioides and X. bisporus at ∼0.656 aw (that is, ≤0.074 and 0.125 mm per day, respectively) were two orders of magnitude slower than those recorded under optimal conditions, yet strains of both species were able to grow down to 0.640 aw (Figures 3 and 4), a hitherto unprecedented limit for mycelial growth of xerophiles. Indeed, at 0.640 aw, A. penicillioides strain JH06THH and X. bisporus strain FRR 3443 grew at rates equivalent to 3.43 and 13.0 mm per year, respectively. In the context of the high-solute and desiccated habitats of xerophiles (some of which can, over periods spanning years or decades, establish populations in environments such as dust and ancient papers and fabrics; Samson and Lustgraaf, 1978; Arai, 2000; Takatori, 2001; Sterflinger and Pinzari, 2012) this is a remarkable rate of growth. Extrapolations indicate lower water-activity limits of 0.636 and 0.632 for X. bisporus FRR 3443 and A. penicillioides JH06THJ, respectively (Supplementary Table S1 and Figures 3 and 4).
The lowest empirically determined water activity for vegetative growth of a eukaryotic microbe is, therefore, 0.640 and the theoretical lower limit is 0.632 (Figure 4); for Archaea or Bacteria the lowest empirically determined value is 0.635, with theoretically determined minima of down to 0.611 (Figures 1 and 2). Planktonic growth of the yeast Zygosaccharomyces rouxii was recorded at 0.620 aw (with a theoretical limit of 0.616; von Schelhorn, 1950), conidial germination of Aspergillus echinulatus at 0.620 (Snow, 1949) and germination of X. bisporus at 0.605 (Pitt and Christian, 1968), although neither development of mycelium nor sporulation of X. bisporus occurred at 0.605 (and none of these achievements have been equalled or exceeded during the subsequent decades, to our knowledge).
No single domain of life is superior in its tolerance of high-solute substrates
A comparison of 60 of the most solute-tolerant microbes (Figure 5) indicates modest water-activity limits for osmotolerant Bacteria and halotolerant/philic eukaryotes relative to those for halophilic Archaea and Bacteria or xerophilic eukayotes. Empirical determinations of water-activity minima for halophilic prokaryotes indicate that numerous strains are not capable of multiplication below 0.755 aw; but that some can do so below 0.650 (Figure 5; Javor, 1984). Up to now, there has been a paucity of studies carried out to establish the true water-activity windows of extreme halophiles by circumventing the solubility limit of NaCl (for example, Table 1 and Figure 1a). Nevertheless, the theoretical determinations suggest parity between the most xerophilic members of the Archaea, Bacteria and Eukarya that are virtually equivalent in their water-activity limits (Figure 5). It is noteworthy that halophilic Archaea and Bacteria are active at water-activity values that are less than both the previously established (Pitt and Christian, 1968; Williams and Hallsworth, 2009) and revised limits for hyphal growth of fungi (0.632, Figure 3) on high-sugar media (Figure 5). If theoretical values (those derived by extrapolation) are not included, the water-activity minimum for multiplication of haloarchaeal strains GN-2 and GN-5 (0.635 aw) are marginally lower than those for hyphal growth of xerophilic fungi (Figures 3 and 4). In nature, extremely halophilic Archaea and Bacteria are not only found in salterns but are also present within, and can dominate, microbial communities located in the hypersaline fluid inclusions of salt crystals in evaporite deposits that underlie a considerable portion of the Earth’s surface (McGenity et al., 2000; Grant, 2004; Gramain et al. 2011). The osmophilic fungal xerophiles X. bisporus and Z. rouxii (see Figures 4 and 5) inhabit high-sugar environments such as dried fruits (for references, see Lievens et al., 2014). Highly xerophilic strains of A. penicillioides, Aspergillus echinulatus, Eurotium amstelodami and Eurotium chevalieri have been isolated from both high-solute and other desiccated habitats (Arai, 2000; Williams and Hallsworth, 2009; Sterflinger and Pinzari, 2012). Eurotium species, Bettsia fastidia, A. penicillioides and W. sebi are particularly common in grains, nuts and spices and, indeed, A. penicillioides may be the pioneer species in such habitats (Hocking 2003; Pitt and Hocking 2009). These fungi are also common spoilage species in many low water-activity baked foods, dried meats and fish, whereas Xerochrysium xerophilum, Eurotium repens, Eurotium halophilicum and W. sebi species are most commonly associated with high-sugar habitats, particularly confectionery, chocolate, jams, maple syrup, dried substrates such as hay, dry beans and grains, and dried fruits (Pitt and Hocking, 2009; Pitt et al., 2013). Water activity can act as a determinant of community composition and ecosystem function for diverse ecophysiological groups (Grant, 2004; Auguet et al., 2010; Herrera et al., 2012; Bolhuis et al., 2013; Cray et al., 2013a; Zajc et al., 2013). We investigated the limits for algae, fungi and nanoflagellates in saline substrates (Supplementary Table S2) and those for Bacteria in high-sugar or high-polyol substrates (Supplementary Table S3).
The water-activity minima for most halophilic members of the Eukarya ranged between 0.743 and 0.712 (for the fungi Polypaecilium pisce at 0.741, Wallemia ichthyophaga at 0.720 and Basipetospora halophila at 0.712; for the algae Dunaliella peircei at 0.743 and Dunaliella salina at 0.739; Supplementary Table S2) that is substantially higher than those of comparable species of halophilic Archaea and Bacteria (Figure 5). Basipetospora halophila and Wallemia ichthyophaga are commonly found in salty environments. Most isolates of Basipetospora halophila have been isolated from salted and dried fish and also from dried seaweed food and sea salt (Pitt and Hocking, 2009). Wallemia ichthyophaga occurs on salted, dried meat, hypersaline waters of salterns (Zalar et al., 2005), salt crystals and MgCl2-rich bitterns (Jančič et al., unpublished data). The hypersaline waters of salterns are also an important habitat for xerophylic Wallemia sebi and Wallemia muriae (Zalar et al., 2005). Dunaliella species are highly prevalent in microbial communities of salt-saturated salterns as well as other niche habitats such as spider-web silk in desert environments (Elevi Bardavid et al., 2008; Azúa-Bustos et al., 2010; Khemakhem et al., 2010; Cray et al., 2013a). The water-activity limits for three nanoflagellates that are also found in salterns at or close to saturated NaCl (0.782 for Euplaesiobystra hypersalinica; 0.767 for Pleurostomum flabellatum; and 0.757 for Halocafeteria seosinensis; see Supplementary Table S2) are lower than those of some other eukaryotes, but are nevertheless exceptional for grazing species that are thought to be absent from most salt-saturated habitats (Elevi Bardavid et al., 2008; Cray et al., 2013a). The xerotolerance of the most sugar-tolerant Bacteria (in the range 0.849 to 0.800 aw for Mycobacterium parascrofulaceum, Mycobacterium smegmatis, Saccharibacter floricola and Tetragenococcus halophilus) was inferior to that for the nanoflagellates E. hypersalinica, H. seosinensis and P. flabellatum as well as fungal comparators (Figure 5). Mycobacterium species, including those that were capable of growth at high concentrations of glycerol or PEG 400 (down to 0.800; Supplementary Table S3; Santos et al., unpublished data), can be isolated from the surface film of sphagnum moss, algal communities and other habitats that (like salterns) may have high concentrations of glycerol and/or other organic low molecular mass solutes that reduce water activity (Walker et al., 2005; Burkholder et al., 2007; Elevi Bardavid et al., 2008; Kazda and Falkinham, 2009). There are various low water-activity, sugar-rich substances of biotic origin such as dried or high-sugar fruits, honey, maple syrup and sugar-beet juice that can delay or prevent microbial colonisation and the formation of microbial biomass as they depress water activity to values outside the growth windows of most, if not all, osmotolerant and osmophilic Bacteria and yeasts (they may also contain antimicrobials and/or constituents—such as fructose and ethanol—that are chaotropic; for references, see Lievens et al., 2014). It is noteworthy that the data presented for biotic activity at extremely low water-activity (Figure 5) come exclusively from culture-dependent studies as there is a paucity of evidence from culture-independent studies to demonstrate microbial processes at equally low water-activities.
Concluding remarks
The findings demonstrate that some species of halophilic Archaea and Bacteria are active at water activities considerably below 0.755, suggesting that—based on extant data sets—microbial xerophilicity ultimately converges on a narrow range of water activity (∼0.650–0.600), and possibly even a common value—of ∼0.61—for all three domains of life. Given that saline, rather than sugar-rich, habitats were most common on the early Earth, this finding has implications for the origins of terrestrial life (Stevenson et al., 2014). Furthermore, we know much about the water activity of potential microbial habitats in extraterrestrial locations, some of which could potentially be inhabited by prokaryotic halophiles capable of multiplication below 0.755 aw (Stevenson et al., 2014). There are implications of the findings of the current study, therefore, in relation to planetary protection (Kminek et al., 2010, 2014; Rummel et al., 2014; Stevenson et al., 2014). The net effect of multiple physiological factors and diverse stress parameters is known to determine the extent of microbial growth windows (Hallsworth, 1998; Hallsworth et al., 2003a, 2007; Williams and Hallsworth, 2009; Bhaganna et al., 2010; Chin et al., 2010; Bell et al., 2013; Harrison et al., 2013; Yakimov et al., 2014). Conversely, it appears that water activity is the ultimate determinant for biotic activity and cell division of numerous extremophiles (for example, haloarchaeal strains GN-2 and GN-5, A. penicillioides and X. bisporus; Figures 1b and 3) and is likely to be equally true for such microbes in their natural habitats, possibly even for microbes not located in high-solute habitats or those that access water from the vapour phase (Rummel et al., 2014; Stevenson et al., 2014). Given the fundamental roles of water as a ‘chaperone’ (McCammick et al., 2010) and in macromolecular hydration, generation of hydrophobic forces, and other interactions within and between cellular macromolecules (Franks, 1972; Daniel et al., 2004; Hallsworth et al., 2007; Bhaganna et al., 2010; Ball, 2012; Cray et al. 2013b), we consider water to be the most potent force to shape the functional biosphere on Earth (see also Pitt, 1975; Brown, 1976, 1990; Hallsworth et al., 2003b; Grant, 2004; Hallsworth et al., 2007; Williams and Hallsworth, 2009; Cray et al., 2013a; Yakimov, et al., 2014). Converging lines of evidence indicate that the ultimate limits for solute tolerance of xerophilic fungi and halophilic Archaea and Bacteria are determined by a prohibitively high energy expenditure that is required for stress adaptation (Hocking, 1993; Oren, 1999; Park et al., 2006; Arino et al., 2010; Cray et al., 2013a). It is therefore noteworthy that Aspergillus species, which appear to have an extraordinary capacity for energy generation (Flipphi et al., 2009; Cray et al., 2013a), also have exceptional tolerances to diverse stresses and an ability to out-compete other microbes, thereby dominating their respective habitats (Cray et al., 2013a). In other words, common physicochemical and/or thermodynamic constraints determine this limit, irrespective of phylogeny.
It may be that a greater understanding of microbial stress biology in relation to low water-activity habitats can lead to further improvements in food preservation, biological control (Cray et al., 2015), management of soil microbiology in arid regions and interventions to enhance crop plant/mycorrhizae (and other plant/microbe) interactions or plant–insect interactions mediated by osmophilic microbes inhabiting plant nectar (Raguso, 2004; Vannette et al., 2012; Herrera et al., 2013; Good et al., 2014); thereby enhancing plant conservation, crop production and, ultimately, global food security. The majority of studies carried out to determine limits of microbial solute tolerance have focused on culturable species of weed-like and/or copiotrophic microbes (Figures 1, 2, 3; Cray et al., 2013a; Oren and Hallsworth, 2014). As a consequence, little is known about water-activity limits for oligotrophic and/or slow-growing species that may undergo a single cell division over a period of decades or longer (Parkes et al., 2000; Lomstein et al., 2012; Rummel et al., 2014). Other questions remain outstanding, such as: what are the metabolic factors that ultimately limit energy generation as well as the synthesis and retention of compatible solutes; can in vitro studies of transcription (Youseff et al., 2014) or culture-independent techniques to detect metabolic activity (Hallsworth et al., 2007; Mosier et al., 2013; Yakimov et al., 2014) offer insights into the in-situ water-activity limits for microbial communities in hostile environments; why are halophilic eukaryotes and osmotolerant/philic prokaryotes relatively intolerant to high solute-concentrations; what are the phenotypic differences between strains of a single bacterial species isolated from salt-rich environments, that are unable to grow at high-sugar concentrations, and those isolated from sugar-rich substrates that can do so (Justé et al., 2008a, 2008b); how do the limitations of certainty and accuracy of water-activity quantification (Stevenson et al., 2014) compare with those of chaotropicity (Cray et al., 2013b), pH and temperature determination in relation to the sensitivity of the cellular system; can further manipulations of environmental chemistry (e.g. Harrison et al., 2014) lead to microbial life at solute concentrations currently thought to be prohibitive; and could synthetic biology be utilised to obtain microbial cell(s) capable of completing life cycles under hitherto nonpermissive conditions? We are intrigued to see whether further studies to address some of these questions can potentially lead to the documentation of life processes at <0.600 aw.
References
Anderson H . (1954). The reddening of salted hides and fish. Appl Microbiol 2: 64–69.
Andrews S, Pitt JI . (1987). Further studies on the water relations of xerophilic fungi, including some halophiles. J Gen Microbiol 133: 233–238.
Antón J, Rosselló-Mora R, Rodríguez-Valera F, Amann R . (2000). Extremely halophilic Bacteria in crystallizer ponds from solar salterns. Appl Environ Microbiol 66: 3052–3057.
Arai H . (2000). Foxing caused by fungi: twenty-five years of study. Int Biodeter Biodegr 46: 181–188.
Arino J, Ramos J, Sychrova H . (2010). Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev 74: 95–120.
Auguet JC, Barberan A, Casamayor EO . (2010). Global ecological patterns in uncultured Archaea. ISME J 4: 182–190.
Ayerst G . (1969). The effects of moisture and temperature on growth and spore germination in some fungi. J Stored Prod Res 5: 127–141.
Azúa-Bustos A, González-Silva C, Salas L, Palma RE, Vicuña R . (2010). A novel subaerial Dunaliella species growing on cave spiderweds in the Atacama Desert. Extremophiles 14: 443–452.
Baker-Austin C, Dopson M . (2007). Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15: 165–171.
Balashov SP, Imasheva ES, Boichenko VA, Antón J, Wang JM, Lanyi JK . (2005). Xanthorhodopsin: a proton pump with a light-harvesting carotenoid antenna. Science 309: 2061–2064.
Ball P . (2012). Concluding remarks: Cum grano salis. Faraday Discuss. 160: 405–414.
Ball P, Hallsworth JE . (2014). Chaotropicity: its uses and abuses. Phys Chem Chem Phys (in press).
Bell ANW, Magill E, Hallsworth JE, Timson DT . (2013). Effects of alcohols and compatible solutes on the activity of β-galactosidase. Appl Biochem Biotech 169: 786–796.
Bhaganna P, Volkers RJM, Bell ANW, Kluge K, Timson DJ, McGrath JW et al. (2010). Hydrophobic substances induce water stress in microbial cells. Microb Biotechnol 3: 701–716.
Bodaker I, Sharon I, Suzuki MT, Feingersch R, Shmoish M, Andreischeva E et al. (2010). Comparative community genomics in the Dead Sea: an increasingly extreme environment. ISME J 4: 399–407.
Bolhuis H, Poele EM, Rodriguez-Valera F . (2004). Isolation and cultivation of Walsby’s square archaeon. Environ Microbiol 6: 1287–1291.
Bolhuis H, Fillinger L, Stal LJ . (2013). Coastal microbial mat diversity along a natural salinity gradient. PLoS One 8: e63166.
Brown AD . (1976). Microbial water stress. Bacteriol Rev 40: 803–846.
Brown AD . (1990). Microbial Water Stress Physiology: Principles and Perspectives. John Wiley and Sons: Chichester, UK.
Burkholder JM, Hallegraeff GM, Melia G, Cohen A, Bowers HA, Oldach DW et al. (2007). Phytoplankton and bacterial assemblages in ballast water of U.S. military ships as a function of port of origin, voyage time, and ocean exchange practices. Harmful Algae 6: 486–518.
Butinar L, Zalar P, Frisvad JC, Gunde-Cimerman N . (2005). The genus Eurotium – members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol Ecol 51: 155–166.
Caurie M . (2005). Water activity of multicomponent mixture of solutes and non-solutes. Int J Food Sci Tech 40: 295–303.
Cayol J–L, Ollivier B, Patel BKC, Ageron E, Grimont PAD, Prensier G et al. (1995). Haloanaerobium lacusroseus sp. nov., an extremely halophilic fermentative bacterium from the sediments of a hypersaline lake. Int J Syst Bacteriol 45: 790–797.
Chin JP, Megaw J, Magill CL, Nowotarski K, Williams JP, Bhaganna P et al. (2010). Solutes determine the temperature windows for microbial survival and growth. Proc Natl Acad Sci USA 107: 7835–7840.
Cifuentes AS, González MA, Inostroza I, Aguilera A . (2001). Reappraisal of physiological attributes of nine strains of Dunaliella (Chlorophyceae): growth and pigment content across a salinity gradient. J Phycol 37: 334–344.
Cowan DA, Tow LA . (2004). Endangered Antarctic microbial communities. Annu Rev Microbiol 58: 649–690.
Cray JA, Bell ANW, Bhaganna P, Mswaka AY, Timson DJ, Hallsworth JE . (2013a). The biology of habitat dominance; can microbes behave as weeds? Microb Biotechnol 6: 453–492.
Cray JA, Russell JT, Timson DJ, Singhal RS, Hallsworth JE . (2013b). A universal measure of chaotropicity and kosmotropicity. Environ Microbiol 15: 287–296.
Cray JA, Bhaganna P, Singhal RS, Patil SV, Saha D, Chakraborty R et al. (2014). Chaotropic and hydrophobic stress mechanisms of antifungal substances. In: Dehne HW, Deising HB, Fraaije B, Gisi U, Hermann D, Mehl A, Oerke EC, Russell PE, Stammler G, Kuck KH, Lyr H, (eds) Modern Fungicides and Antifungal Compounds vol. VII. Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany. ISBN: 978-3-941261-13-6.
Cray JA, Houghton JDR, Cooke LR, Hallsworth JE . (2015). A simple inhibition coefficient for quantifying potency of biocontrol agents against plant-pathogenic fungi. Biol Control 81: 93–100.
Daffonchio D, Borin S, Brusa T, Brusetti L, van der Wielen PWJJ, Bolhuis H et al. (2006). Stratified prokaryote network in the oxic-anoxic transition of a deep-sea halocline. Nature 440: 203–207.
Daniel RM, Finney JL, Stoneham M . (2004). The molecular basis of life: is life possible without water? A discussion meeting held at the Royal Society, London, UK, 3-4 December 2003. Philos Trans R Soc London B Biol Sci 359: 1141–1328.
Deole R, Challacombe J, Ralford DW, Hoff WD . (2013). An extremely halophilic proteobacterium combines a highly acidic proteome with a low cytoplasmic potassium content. J Biol Chem 288: 581–588.
Elevi Bardavid R, Khristo P, Oren A . (2008). Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles 12: 5–14.
Ferrer M, Chernikova TN, Yakimov MM, Golyshin PN, Timmis KN . (2003). Chaperonins govern growth of Escherichia coli at low temperatures. Nat Biotechnol 21: 1266–1267.
Ferro Fontán C, Chirife J . (1981). The evaluation of water activity in aqueous-solutions from freezing-point depression. J Food Technol 16: 21–30.
Flipphi M, Sun JB, Robellet X, Karaffa L, Fekete E, Zeng AP et al. (2009). Biodiversity and evolution of primary carbon metabolism in Aspergillus nidulans and other Aspergillus spp. Fungal Genet Biol. 46: S19–S44.
Franks F . (1972) Water: A Comprehensive Treatise. Plenum: New York, USA.
Gock MA, Hocking AD, Pitt JI, Poulos PG . (2003). Influence of temperature, water activity and pH on growth of some xerophilic fungi. Int J Food Microbiol. 81: 11–19.
Golyshina OV . (2011). Environmental, biogeographic, and biochemical patterns of Archaea of the family Ferroplasmaceae. Appl Environ Microbiol 77: 5071–5078.
Gramain A, Díaz GC, Demergasso C, Lowenstein TK, McGenity TJ . (2011). Achaeal diversity along a subterranean salt core from the Salar Grande (Chile). Environ Microbiol 13: 2105–2121.
Grant WD . (2004). Life at low water activity. Philos Trans R Soc London B Biol Sci 359: 1249–1266.
Good AP, Gauthier LPL, Vannette RL, Fukami T . (2014). Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS One 9: e86494.
Greenspan L . (1977). Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand A Phys Chem 81A: 89–96.
Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ et al. (2004). Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum. Extremophiles 8: 431–439.
Hallsworth JE . (1998). Ethanol-induced water stress in yeast. J Ferment Bioeng 85: 125–137.
Hallsworth JE, Heim S, Timmis KN . (2003a). Chaotropic solutes cause water stress in Pseudomonas putida. Environ Microbiol 5: 1270–1280.
Hallsworth JE, Magan N . (1994a). Effect of carbohydrate type and concentration on polyols and trehalose in conidia of three entomopathogenic fungi. Microbiology 140: 2705–2713.
Hallsworth JE, Magan N . (1994b). Effects of KCl concentration on accumulation of acyclic sugar alcohols and trehalose in conidia of three entomopathogenic fungi. Lett Appl Microbiol 18: 8–11.
Hallsworth JE, Magan N . (1994c). Improved biological control by changing polyols/trehalose in conidia of entomopathogens. In: Brighton Crop Protection Council-Pests and Diseases. British Crop Protection Council 1994: Farnham, UK, pp 1091–1096.
Hallsworth JE, Magan N . (1995). Manipulation of intracellular glycerol and erythritol enhances germination of conidia at low water availability. Microbiology 141: 1109–1115.
Hallsworth JE, Magan N . (1996). Culture age, temperature and pH affect the polyol and trehalose contents of fungal propagules. Appl Environ Microbiol 62: 2435–2442.
Hallsworth JE, Magan N . (1999). Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J Invertebr Pathol 74: 261–266.
Hallsworth JE, Nomura Y . (1999). A simple method to determine the water activity of ethanol-containing samples. Biotechnol Bioeng 62: 242–245.
Hallsworth JE, Nomura Y, Iwahara M . (1998). Ethanol-induced water stress and fungal growth. J Ferment Bioeng 86: 451–456.
Hallsworth JE, Prior BA, Nomura Y, Iwahara M, Timmis KN . (2003b). Compatible solutes protect against chaotrope (ethanol)-induced, nonosmotic water stress. Appl Environ Microbiol 69: 7032–7034.
Hallsworth JE, Yakimov MM, Golyshin PN, Gillion JLM, D'Auria G, Alves FL et al. (2007). Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol 9: 803–813.
Halpern M, Fridman S, Atamna-Ismaeel N, Izhaki I . (2013). Rosenbergiella nectarea gen nov. sp. nov., in the family Enterobacteriaceae, isolated from floral nectar. Int J Syst Evol Microbiol 63: 4259–4265.
Harrison JP, Gheeraert N, Tsigelnitskiy D, Cockell CS . (2013). The limits for life under multiple extremes. Trends Microbiol 21: 204–212.
Harrison JP, Hallsworth JE, Cockell CS . (2014). Iron starvation combined with microaerobic conditions reduces the temperature sensitivity of Halomonas hydrothermalis. Appl Environ Microbiol (in press).
Herrera CM, Pozo MI, Bazaga P . (2012). Jack of all nectars, master of most: DNA methylation and the epigenetic basis of niche width in a flower-living yeast. Mol Ecol 21: 2602–2616.
Herrera CM, Pozo MI, Medrano M . (2013). Yeasts in nectar of an early-blooming herb: sought by bumble bees, detrimental to plant fecundity. Ecology 94: 273–279.
Hocking AD . (1993). Responses of xerophilic fungi to changes in water activity. In: Jennings DH, (ed). Stress Tolerance of Fungi. Marcel Decker: New York, USA, pp 233–256.
Hocking AD . (2003). Microbiological facts and fictions in grain storage. In: Wright EJ, Webb MC, Highley E, (eds.) Stored Grain in Australia. Proceedings of the Australian Postharvest Technical Conference. CSIRO: Canberra, pp 55–58.
Huang MR, Li SX, Dong ZQ, Feng W, Wang XY, Gu SY et al. (2002). Oxygen enrichment from air through multilayer thin low-density polyethylene films. J Appl Polymer Sci 83: 3013–3021.
Huchet V, Pavan S, Lochardet A, Divanac’h ML, Postollec F, Thualt D . (2013). Development and application of a predictive model of Aspergillus candidus growth as a tool to improve shelf life of bakery products. Food Microbiol 36: 254–259.
Imhoff JF, Süling J . (1996). The phylogenetic relationship among Ectothiorhodospiraceae: a reevaluation of their taxonomy on the basis of 16S rDNA analyses. Arch Microbiol 165: 106–113.
Ivanov IT . (2001). Rapid method for comparing the cytotoxicity of organic solvents and their ability to destabilize proteins of the erythrocyte membrane. Pharmazie 56: 808–809.
Javor BJ, Requadt C, Stoeckenius W . (1982). Box-shaped halophilic bacteria. J Bacteriol 151: 1532–1542.
Javor BJ . (1984). Growth potential of halophilic bacteria isolated from solar salt environments: carbon sources and salt requirements. Appl Environ Microbiol 48: 352–360.
Javor BJ . (1989) Hypersaline Environments: Microbiology and Biogeochemistry. Springer-Verlag: Berlin.
Jojima Y, Mihara Y, Suzuki S, Yokozeki K, Yamanaka S, Fudou R . (2004). Saccharibacter floricola gen. nov., sp. nov., a novel osmophilic acetic acid bacterium isolated from pollen. Int J Syst Evol Micr 54: 2263–2267.
Justé A, Lievens B, Klingeberg M, Michiels CW, Marsh TL, Willems KA . (2008a). Predominance of Tetragenococcus halophilus as the cause of sugar thick juice degradation. Food Microbiol 25: 413–421.
Justé A, Lievens B, Frans I, Marsh TL, Klingeberg M, Michiels CW et al. (2008b). Genetic and physiological diversity of Tetragenococcus halophilus strains isolated from sugar- and salt-rich environments. Microbiology 154: 2600–2610.
Kar JR, Hallsworth JE, Singhal RS . (2014). Fermentative production of glycine betaine and trehalose from acid whey using Actinopolyspora halophila (MTCC 263). Environ Technol Innovat (in press).
Kashangura C, Hallsworth JE, Mswaka AY . (2006). Phenotypic diversity amongst strains of Pleurotus sajor-caju: implications for cultivation in arid environments. Mycol Res 110: 312–317.
Kashefi K, Lovley DR . (2003). Extending the upper temperature limit for life. Science 301: 934–934.
Kazda J, Falkinham JO III . (2009). Mycobacteria in sphagnum, peats and potting soils. In: Kazda J, Pavlik I, Falkinham JO III, Hruska K, (eds) The Ecology of Mycobacteria: Impact on Animal’s and Human’s Health. Springer: Heidelberg, pp 89–95.
Khemakhem H, Elloumi J, Moussa M, Aleya L, Ayadi H . (2010). The concept of ecological succession applied to phytoplankton over four consecutive years in five ponds featuring a salinity gradient. Estuar Coast Shelf Sci 88: 33–44.
Kminek G, Rummel JD, Cockell CS, Atlas R, Barlow N, Beaty D et al. (2010). Report of the COSPAR Mars special regions colloquium. Adv Space Res 46: 811–829.
Kminek G, Conley C, Allen CC, Bartlett DH, Beaty DW, Benning DG et al. (2014). Report of the workshop for life detection on samples from Mars. Life Sci Space Res 2: 1–5.
Kocur M, Hodgkiss W . (1973). Taxonomic status of the genus Halococcus Schoop. Int J Syst Evol Microbiol 23: 151–156.
Krisko A, Radman M . (2013). Biology of extreme radiation resistance: the way of Deinococcus radiodurans. Cold Spring Harb Perspect Biol 5: 1–11.
Leong SL, Pettersson OV, Rice T, Hocking AD, Schnürer J . (2011). The extreme xerophilic mould Xeromyces bisporus – growth and competition at various water activities. Int J Food Microbiol 145: 57–63.
Lievens B, Hallsworth JE, Belgacem ZB, Pozo MI, Stevenson A, Willems KA et al. Microbiology of sugar-rich environments: diversity, ecology, and system constraints. Environ Microbiol 2014; e-pub ahead of print 3 September 2014; doi:10.1111/1462-2920.12570.
Lomstein BA, Langerhuus AT, D'Hondt S, Jørgensen BB, Spivack AJ . (2012). Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484: 101–104.
Manzoni S, Schimel JP, Porporato A . (2012). Responses of soil microbial communities to water-stress: results from a meta-analysis. Ecology 93: 930–938.
McCammick EM, Gomase VS, Timson DJ, McGenity TJ, Hallsworth JE . (2010). Water-hydrophobic compound interactions with the microbial cell. In: Timmis KN, (ed) Handbook of Hydrocarbon and Lipid Microbiology – Hydrocarbons, Oils and Lipids: Diversity, Properties and Formation vol. 2. Springer: New York, USA, pp 1451–1466.
McGenity TJ, Gemmell RT, Grant WD, Stan-Lotter H . (2000). Origins of halophilic microorganisms in ancient salt deposits. Environ Microbiol 2: 243–250.
Mosier AC, Justice NB, Bowen BP, Baran R, Thomas BC, Northen TR et al. (2013). Metabolites associated with adaptation of microorganisms to an acidophilic, metal-rich environment identified by stable-isotope-enabled metabolomics. mBio 4: e0048412.
Moyano FE, Manzoni S, Chenu C . (2013). Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models. Soil Biol Biochem 59: 72–85.
Neumeyer K, Ross T, McMeekin TA . (1997). Development of a predictive model to describe the effects of temperature and water activity on the growth of spoilage pseudomonads. Int J Food Microbiol 38: 45–54.
Norrish RS . (1966). An equation for the activity coefficients and equilibrium relative humidities of water in confectionery syrups. Int J Food Sci Tech 1: 25–39.
Norton CF, McGenity TJ, Grant WD . (1993). Archaeal halophiles (halobacteria) from two British salt mines. J Gen Microbiol 139: 1077–1081.
Ntougias S, Zervakis GI, Fasseas C . (2007). Halotalea alkalilenta gen. nov., sp. nov., a novel osmotolerant and alkalitolerant bacterium from alkaline olive mill wastes, and emended description of the family Halomonadaceae Franzmann et al. 1989, emend. Dobson and Franzmann 1996. Int J Syst Evol Microbiol 57: 1975–1983.
Ollivier B, Caumette P, Garcia JL, Mah RA . (1994). Anaerobic bacteria from hypersaline environments. Microbiol Rev 58: 27–38.
Oren A . (1999). Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63: 334–348.
Oren A . (2000). Change of the names Haloanaerobiales, Haloanaerobiaceae and Haloanaerobium to Halanaerobiales, Halanaerobiaceae and Halanaerobium, respectively, and further nomenclatural changes within the order Halanaerobiales. Int J Syst Evol 50: 2229–2230.
Oren A . (2013). Life in magnesium- and calcium-rich hypersaline environments: salt stress by chaotropic ions. In: Seckbach J, Oren A, Stan-Lotter H, (eds) Polyextremophiles: Life Under Multiple Forms of Stress. Cellular Origin, Life in Extreme Habitats and Astrobiology 27 pp. 217–232. Springer: Dordrecht, The Netherlands.
Oren A, Hallsworth JE . (2014). Microbial weeds in saline habitats: the enigma of the weed-like Haloferax mediterranei. FEMS Microbiol Lett 359: 134–142.
Oren A, Heldal M, Norland S, Galinski EA . (2002). Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles 6: 491–498.
Park JS, Cho BC, Simpson AGB . (2006). Halocafeteria seosinensis gen. et sp. nov. (Bicosoecida), a halophilic bacterivorous nanoflagellate isolated from a solar saltern. Extremophiles 10: 493–504.
Park JS, Simpson AG, Lee WJ, Cho BC . (2007). Ultrastructure and phylogenetic placement within Heterolobosea of the previously unclassified, extremely halophilic heterotrophic flagellate Pleurostomum flabellatum (Ruinen 1938). Protist 159: 397–413.
Park JS, Simpson AG, Brown S, Cho BC . (2009). Ultrastructure and molecular phylogeny of two heterolobosean amoebae, Euplaesiobystra hypersalinica gen. et sp. nov. and Tulamoeba peronaphora gen. et sp. nov., isolated from an extremely hypersaline habitat. Protist 160: 265–283.
Parkes RJ, Cragg BA, Wellsbury P . (2000). Recent studies on bacterial populations and processes in subseafloor sediments: a review. Hydrogeol J 8: 11–28.
Payne JI, Sehgal SN, Gibbons NE . (1960). Immersion refractometry of some halophilic bacteria. Can J Microbiol 6: 9–15.
Peña A, Teeling H, Huerta-Cepas J, Santos F, Yarza P, Brito-Echeverría J et al. (2010). Fine-scale evolution: genomic, phenotypic and ecological differentiation in two coexisting Salinibacter ruber strains. ISME J. 4: 882–895.
Pirt SJ . (1975) Stoichiometry and Kinetics of Microbial Growth: Bioenergetics of Microbial Growth and Product Formation. Pirtferm Limited: London, UK.
Pitt JI . (1975). Xerophilic fungi and the spoilage of foods of plant origin. In: Duckworth RB, (ed) Water Relations of Foods. Academic Press: London, UK, pp 273–307.
Pitt JI, Christian JHB . (1968). Water relations of xerophilic fungi isolated from prunes. Appl Environ Microbiol 16: 1853–1858.
Pitt JI, Hocking AD . (1977). Influence of solute and hydrogen ion concentration on the water relations of some xerophilic fungi. J Gen Microbiol 101: 35–40.
Pitt JI, Hocking AD . (2009). Aspergillus. Fungi and Food Spoilage 3rd edn Springer: New York, USA.
Pitt JI, Lantz H, Petterson OV, Leong SL . (2013). Xerochrysium gen. nov. and Bettsia, genera encompassing xerophilic species of Chrysosporium. IMA Fungus 4: 229–241.
Potts M . (1994). Desiccation tolerance of prokaryotes. Microbiol Rev 58: 755–805.
Raguso RA . (2004). Why are some floral nectars scented? Ecology 85: 1486–1494.
Rosso L, Robinson TP . (2001). A cardinal model to describe the effect of water activity on the growth of moulds. Int J Food Microbiol 63: 265–273.
Ruan JS, Al-Tai AM, Zhou ZH, Qu LH . (1994). Actinopolyspora iraqiensis sp. nov., a new halophilic actinomycete isolate from soil. Int J Syst Bacteriol 44: 759–763.
Rummel JD, Beaty DW, Jones MA, Bakermans C, Barlow NG, Boston P et al. (2014). A new analysis of Mars ‘Special Regions’, findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14: 887–968.
Samson RA, Lustgraaf BVD . (1978). Aspergillus penicilloides and Eurotium halophilicum in association with house-dust mites. Mycopathologia 64: 13–16.
Sass AM, McKew BA, Sass H, Fichtel J, Timmis KN, McGenity TJ . (2008). Diversity of Bacillus-like organisms isolated from deep-sea hypersaline anoxic sediments. Saline Systems 4: 8.
Sher J, Elevi R, Mana L, Oren A . (2004). Glycerol metabolism in the extremely halophilic bacterium Salinibacter ruber. FEMS Microbiol Lett. 19: 211–215.
Snow D . (1949). The germination of mould spores at controlled humidities. Ann Appl Biol 36: 1–13.
Sterflinger K, Pinzari F . (2012). The revenge of time: fungal deterioration of cultural heritage with particular reference to books, paper and parchment. Environ Microbiol. 14: 559–566.
Stevenson A, Hallsworth JE . (2014). Water and temperature relations of soil Actinobacteria. Environ Microbiol Rep 6: 744–755.
Stevenson A, Burkhardt J, Cockell CS, Cray JA, Dijksterhuis J, Fox-Powell M et al. Multiplication of microbes below 0.690 water activity: implications for terrestrial and extraterrestrial life. Environ Microbiol 2014; e-pub ahead of print 28 September 2014; doi:10.1111/1462-2920.12598.
Takatori K . (2001). Fungal allergy - fungal ecology in dwelling environments. Nihon Ishinkin Gakkai Zasshi 42: 113–117.
Tang SK, Wang Y, Klenk HP, Shi R, Lou K, Zhang YJ et al. (2011). Actinopolyspora alba sp. nov. and Actinopolyspora erythraea sp. nov., isolated from a salt field, and reclassification of Actinopolyspora iraqiensis Ruan et al. 1994 as a heterotypic synonym of Saccharomonospora halophila. Int J Syst Evol Micr 61: 1693–1698.
Tassou CC, Panagou EZ, Natskoulis P, Magan N . (2007). Modelling the effect of temperature and water activity on the growth of two ochratoxigenic strains of Aspergillus carbonarius from Greek wine grapes. J Appl Microbiol 103: 2267–2276.
Tienungoon S, Ratkowsky DA, McMeekin TA, Ross T . (2000). Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl Environ Microbiol 66: 4979–4987.
Tindall BJ . (1992). The family Halobacteriaceae. In: Balows A, Trüper HG, Dwokin M, Harder W, Schleiger KH (eds) The Prokaryotes. A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identifications, 2nd Ed, Vol I. Springer-Verlag: New York, pp 768–808.
Tomlinson GA, Hochstein LI . (1976). Halobacterium saccharovorum sp. nov., a carbohydrate metabolizing extremely halophilic bacterium. Can J Microbiol 22: 587–591.
Vaisman N, Oren A . (2009). Salisaeta longa gen. nov., sp. nov., a red, halophilic member of the Bacteroidetes. Int J Syst Evol Microbiol 59: 2571–2574.
Valentine DL . (2013). Microbiology: intraterrestrial lifestyles. Nature 496: 176–177.
Vannette RL, Gathier MP, Fukami T . (2012). Nectar bacteria, but not yeast, weaken a plant-pollinator mutualism. Proc Biol Sci 280: 20122601.
von Schelhorn M . (1950). Untersuchungen über den Verderb wasserarmer Lebensmittel durch osmophile Mikroorganismen. II. Grenzkonzentrationen für den osmophilen Schimmelpilz Aspergillus glaucus in Abhängigkeit vom pH Wert des Substrates. Z Lebensm Untersuch Forsch 91: 338–342.
Wainø M, Tindall BJ, Ingvorsen K . (2000). Halorhabdus utahensis gen. nov., sp. nov., an aerobic, extremely halophilic member of the Archaea from Great Salt Lake, Utah. Int J Syst Evol Micr 50: 183–190.
Walker JJ, Spear JR, Pace NR . (2005). Geobiology of a microbial endolithic community in the Yellowstone geothermal environment. Nature 434: 1011–1014.
Whitman WB, Coleman DC, Wiebe WJ . (1998). Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583.
Williams JP, Hallsworth JE . (2009). Limits of life in hostile environments; no limits to biosphere function? Environ Microbiol 11: 3292–3308.
Winston PW, Bates PS . (1960). Saturated salt solutions for the control of humidity in biological research. Ecology 41: 232–237.
Wyatt TT, Golovina EA, van Leeuwen MR, Hallsworth JE, Wösten HAB, Dijksterhuis J Decreases in bulk water and mannitol and accumulation of trehalose and trehalose-based oligosaccharides define a two-stage maturation process towards extreme stress-resistance in ascospores of Neosartorya fischeri (Aspergillus fischeri). Environ Microbiol 2014; e-pub ahead of print 7 October 2014; doi:10.1111/1462-2920.12557.
Yakimov MM, Lo Cono V, La Spada G, Bortoluzzi G, Messina E, Smedile F et al. Microbial community of seawater-brine interface of the deep-sea brine Lake Kryos as revealed by recovery of mRNA are active below the chaotropicity limit of life. Environ Microbiol 2014; e-pub ahead of print 6 August 2014; doi:10.1111/1462-2920.12587.
Yoshida M, Matsubara K, Kudo T, Horikoshi K . (1991). Actinopolyspora mortivallis sp. nov. a moderately halophilic actinomycete. Int J Syst Bacteriol 41: 15–20.
Youseff NH, Savage-Ashlock KN, McCully AL, Luedtke B, Shaw EI, Hoff WD et al. (2014). Trehalose/2-sulfotrehalose biosynthesis and glycine-betaine uptake are widely spread mechanisms for osmoadaptation in the Halobacteriales. ISME J 8: 636–649.
Yu X, Schmidt AR, Schmidt SJ . (2009). Uncertainty analysis of hygrometer-obtained water activity measurements of saturated salt slurries and food materials. Food Chem. 115: 214–226.
Zajc J, Liu Y, Dai W, Yang Z, Hu J, Gostinčar C et al. (2013). Genome and transcriptome sequencing of the halophilic fungus Wallemia ichthyophaga: haloadaptations present and absent. BMC Genomics 14: 617–617.
Zalar P, de Hoog SG, Schroers HJ, Frank JM, Gunde-Cimerman N . (2005). Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie van Leeuwenhoek 87: 311–328.
Acknowledgements
We are grateful to Peter N Golyshin and Olga V Golyshina (Bangor University, UK), Barbara J Javor (Southwest Fisheries Science Center, USA) and Tom L Kieft (New Mexico Tech., USA) for fruitful discussions, and to Helga Stan-Lotter (University of Salzburg, Austria) for providing Halobacterium sp. NRC-1. Funding was received from the Research (Northern Ireland) and Enterprise Directorate of Queen’s University Belfast; Department of Agriculture and Rural Development and the Department for Employment and Learning (Northern Ireland); Biotechnology and Biological Sciences Research Council (BBSRC, UK) Projects BBF/003471/1 and BBF/00351X/1; and the Beaufort Marine Research Award for Marine Biodiscovery that is carried out under the Sea Change Strategy and the Strategy for Science Technology and Innovation (2006–2013), with the support of the Marine Institute, Ireland. This work was also supported by the State of São Paulo Research Foundation (FAPESP) via a grant awarded to Drauzio EN Rangel (#2010/06374-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Stevenson, A., Cray, J., Williams, J. et al. Is there a common water-activity limit for the three domains of life?. ISME J 9, 1333–1351 (2015). https://doi.org/10.1038/ismej.2014.219
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2014.219
This article is cited by
-
Monitoring the growth dynamics of Tetragenococcus halophilus strains in lupine moromi fermentation using a multiplex-PCR system
BMC Research Notes (2023)
-
Water availability creates global thresholds in multidimensional soil biodiversity and functions
Nature Ecology & Evolution (2023)
-
Ecological successions throughout the desiccation of Tirez lagoon (Spain) as an astrobiological time-analog for wet-to-dry transitions on Mars
Scientific Reports (2023)
-
DNA sequencing at the picogram level to investigate life on Mars and Earth
Scientific Reports (2023)
-
Waterless sterilization of clinical solid waste using supercritical carbon dioxide: fungal spores inactivation mechanisms, optimization and artificial neural network models
Biomass Conversion and Biorefinery (2023)