Abstract
Nitrous oxide (N2O) emissions from grazed pastures are a product of microbial transformations of nitrogen and the prevailing view is that these only occur in the soil. Here we show this is not the case. We have found ammonia-oxidising bacteria (AOB) are present on plant leaves where they produce N2O just as in soil. AOB (Nitrosospira sp. predominantly) on the pasture grass Lolium perenne converted 0.02–0.42% (mean 0.12%) of the oxidised ammonia to N2O. As we have found AOB to be ubiquitous on grasses sampled from urine patches, we propose a ‘plant’ source of N2O may be a feature of grazed grassland.
Similar content being viewed by others
Main
In terms of climate forcing, nitrous oxide (N2O) is the third most important greenhouse gas (Blunden and Arndt, 2013). Agriculture is the largest source of anthropogenic N2O (Reay et al., 2012) with about 20% of agricultural emissions coming from grassland grazed by animals (Oenema et al., 2005).
Grazed grassland is a major source of N2O because grazers harvest nitrogen (N) from plants across a wide area but recycle it back onto the pasture, largely as urine, in patches of very high N concentration. The N in urine patches is often in excess of what can be used by plants resulting in losses through leaching as nitrate, as N2O and through volatilisation as ammonia (NH3) creating a high NH3 environment in the soil and plant canopy; an important point that we will return to later. The established wisdom is that N2O is generated exclusively by soil-based microbes such as ammonia-oxidising bacteria (AOB). This soil biology is represented in models designed to simulate N2O emissions and the soil is a target for mitigation strategies such as the use of nitrification inhibitors.
We have previously shown that pasture plants can emit N2O largely through acting as a conduit for emissions generated in the soil, which are themselves controlled to some degree by the plant (Bowatte et al., 2014). In this case the origin of the emission is still the soil microbes. However, AOB have been found on the leaves of plants, for example, Norway spruce (Papen et al., 2002; Teuber et al., 2007) and weeds in rice paddies (Bowatte et al., 2006), prompting us to ask whether AOB might be present on the leaves of pasture species and contribute to N2O emissions as they do in soil.
We looked for AOB on plants in situations where NH3 concentrations were likely to be high, choosing plants from urine patches in grazed pastures and plants from pastures surrounding a urea fertiliser manufacturing plant. DNA was extracted from the leaves (including both the surface and apoplast) and the presence of AOB tested using PCR. AOB were present in all the species we examined—the grasses Lolium perenne, Dactylis glomerata, Anthoxanthum odoratum, Poa pratensis, Bromus wildenowii and legumes Trifolium repens and T. subterraneum.
To measure whether leaf AOB produce N2O, we used intact plants of ryegrass (L. perenne) lifted as cores from a paddock that had been recently grazed by adult sheep. The cores were installed in a chamber system designed to allow sampling of above- and belowground environments separately (Bowatte et al., 2014). N2O emissions were measured from untreated (control) plants and from plants where NH3 was added to the aboveground chamber and leaves were either untreated or sterilised by wiping twice with paper towels soaked in 1% hypoclorite (Sturz et al., 1997) and then with sterile water. We tested for the presence and abundance of AOB on the leaves by extracting DNA and using PCR and real-time PCR targeting the ammonia monoxygenase A (amoA) gene, which is characteristic of AOB. AOB identity was established using cloning and DNA sequencing. Further details of these experiments can be found in the Supplementary Information.
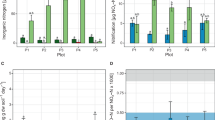
The addition of NH3 to untreated plants significantly stimulated N2O emissions (P<0.001) compared with the controls; by contrast, the plants with sterilised leaves produced significantly less N2O than controls (P<0.001) even with NH3 added (Figure 1) providing strong evidence for emissions being associated with bacteria on the leaves. Control plants did emit N2O suggesting there was either sufficient NH3 available for bacterially generated emissions and/or other plant-based mechanisms were involved (Bowatte et al., 2014).
The major AOB species identified was Nitrosospira strain III7 that has been previously shown to produce N2O (Jiang and Bakken, 1999). We measured 109 AOB cells per m2 ryegrass leaf, assuming a specific leaf area of 250 cm2 g−1 leaf.
The rate of production of N2O (0.1–0.17 mg N2O-N per m2 leaf area per hour) can be translated to a field situation using the leaf area index (LAI)—1 m2 leaf per m2 ground would be an LAI of 1. LAI in a pasture can vary from <1 to >6 depending on the management (for example, Orr et al., 1988). At LAI of 1, the AOB leaf emission rate would equate to a N2O emission rate of about 0.1–0.3 mg N2O-N per m2 ground per hour. By comparison, the emission rates measured after dairy cattle urine (650 kg N ha−1) was applied to freely and poorly drained soil were 0.024–1.55 and 0.048–3.33 mg N2O-N per m2 ground per hour, respectively (Li and Kelliher, 2005).
The fraction of the NH3 that was converted to N2O by the leaf AOB was 0.02–0.42% (mean 0.12%). The mean value is close to that measured for Nitrosospira strains including strain III7 isolated from acidic, loamy and sandy soils where values ranged from 0.07 to 0.10% (Jiang and Bakken, 1999). This is good evidence that the AOB on leaves have the capacity to produce N2O at the same rate as AOB in soils. We do not suggest that leaf AOB will produce as much N2O as soil microbes; however, because leaf AOB have access to a source of substrate—volatilised NH3—that is unavailable to soil microbes and may constitute 26% (Laubach et al., 2013) to 40% (Carran et al., 1982) of the N deposited in the urine, N2O emissions from these aboveground AOB are additional to soil emissions. Further research is required to identify the situations in which leaf AOB contribute to total emissions and to quantify this contribution.
References
Blunden J, Arndt DS . (2013). State of the climate in 2012. B Am Meteorol Soc 94: S1–S258.
Bowatte S, Ishihara R, Asakawa S, Kimura M . (2006). Characterization of ammonia oxidizing bacteria associated with weeds in a Japanese paddy field using amoA gene fragments. Soil Sci Plant Nutr 52: 593–600.
Bowatte S, Newton PCD, Theobald P, Brock S, Hunt C, Lieffering M et al. (2014). Emissions of nitrous oxide from the leaves of grasses. Plant Soil 374: 275–283.
Carran RA, Ball PR, Theobald PW, Collins MEG . (1982). Soil nitrogen balances in urine-affected areas under two moisture regimes in Southland. N Z J Exper Agric 10: 377–381.
Jiang QQ, Bakken LR . (1999). Nitrous oxide production and methane oxidation by different ammonia- oxidizing bacteria. Appl Environ Microbiol 65: 2679–2684.
Laubach J, Taghizadeh-Toosi A, Gibbs SJ, Sherlock RR, Kelliher FM, Grover SPP . (2013). Ammonia emissions from cattle urine and dung excreted on pasture. Biogeosciences 10: 327–338.
Li Z, Kelliher FM . (2005). Determining nitrous oxide emissions from subsurface measurements in grazed pasture: A field trial of alternative technology. Aust J Soil Res 43: 677–687.
Oenema O, Wrage N, Velthof GL, Van Groenigen JW, Dolfing J, Kuikman PJ . (2005). Trends in global nitrous oxide emissions from animal production systems. Nutr Cycl Agroecosys 72: 51–65.
Orr RJ, Parsons AJ, Treacher TT, Penning PD . (1988). Seasonal patterns of grass production under cutting or continuous stocking managements. Grass Forage Science 43: 199–207.
Papen H, Geβler A, Zumbusch E, Rennenberg H . (2002). Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr Microbiol 44: 56–60.
Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F et al. (2012). Global agriculture and nitrous oxide emissions. Nat Clim Change 2: 410–416.
Sturz AV, Christie BR, Matheson BG, Nowak J . (1997). Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol Fertility Soils 25: 13–19.
Teuber M, Papen H, Gasche R, Eßmüller TH, Gebßler A . (2007). The apoplast of norway spruce (Picea abies) needles as habitat and reaction compartment for autotrophic nitrifiers. In: Sattelmacher B, Horst WJ, (eds) The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions. Springer, pp 405–425.
Acknowledgements
This work was supported by AgResearch and the New Zealand Agricultural Greenhouse Gas Research Centre. The authors thank Zhong Lei for plant DNA extraction and real time PCR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Bowatte, S., Newton, P., Brock, S. et al. Bacteria on leaves: a previously unrecognised source of N2O in grazed pastures. ISME J 9, 265–267 (2015). https://doi.org/10.1038/ismej.2014.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2014.118