Abstract
N2O gas is involved in global warming and ozone depletion. The major sources of N2O are soil microbial processes. Anthropogenic inputs into the nitrogen cycle have exacerbated these microbial processes, including nitrification. Ammonia-oxidizing archaea (AOA) are major members of the pool of soil ammonia-oxidizing microorganisms. This study investigated the isotopic signatures of N2O produced by soil AOA and associated N2O production processes. All five AOA strains (I.1a, I.1a-associated and I.1b clades of Thaumarchaeota) from soil produced N2O and their yields were comparable to those of ammonia-oxidizing bacteria (AOB). The levels of site preference (SP), δ15Nbulk and δ18O -N2O of soil AOA strains were 13–30%, −13 to −35% and 22–36%, respectively, and strains MY1–3 and other soil AOA strains had distinct isotopic signatures. A 15N-NH4+-labeling experiment indicated that N2O originated from two different production pathways (that is, ammonia oxidation and nitrifier denitrification), which suggests that the isotopic signatures of N2O from AOA may be attributable to the relative contributions of these two processes. The highest N2O production yield and lowest site preference of acidophilic strain CS may be related to enhanced nitrifier denitrification for detoxifying nitrite. Previously, it was not possible to detect N2O from soil AOA because of similarities between its isotopic signatures and those from AOB. Given the predominance of AOA over AOB in most soils, a significant proportion of the total N2O emissions from soil nitrification may be attributable to AOA.
Similar content being viewed by others
Introduction
Nitrous oxide (N2O) is a long-lived greenhouse gas, which is also involved in ozone depletion. The atmospheric abundance of N2O is approximately one-thousandth of CO2 but its radiative efficiency (Wm−2 ppb−1) is 216 times greater than that of CO2 (Rahn and Wahlen, 1997). Soils are major sources of N2O (Conrad, 1996) and are believed to contribute approximately 65% of the total N2O emitted to the atmosphere (Seitzinger et al., 2000). The atmospheric concentrations of N2O have been increasing from pre-industrial levels of 0.27 ppmv to current concentrations approaching 0.32 ppmv (Conrad, 1996). Nitrogen deposition and nitrogen fertilization (Nevison and Holland, 1997) due to agricultural expansion and intensification have contributed to the conspicuous post-industrial increase in atmospheric N2O loading via increased soil nitrogen availability.
The pathways of biological N2O production include partial dissimilatory nitrate or nitrite reduction (denitrification), nitrifier denitrification (Wrage et al., 2001), ammonia (hydroxylamine) oxidation and NOx detoxification (also known as the ‘nitrosative stress’ pathway) (Hendriks et al., 2000). Nitrification-related pathways (nitrifier denitrification and ammonia oxidation) mediated by ammonia-oxidizing bacteria (AOB) are known to be a major source of N2O production from terrestrial environments (Gödde and Conrad, 1999). The first N2O-yielding route in AOB is related to the activity of hydroxylamine oxidoreductase (HAO), which mediates the conversion of hydroxylamine to nitrite, the second step in ammonia oxidation. The second N2O-yielding route is through a denitrification pathway in AOB, the so-called nitrifier denitrification, where nitrite is reduced to NO and N2O (Wrage et al., 2001; Arp and Stein, 2003), as in the classical heterotrophic denitrification pathway.
Isotopologue analysis of N2O has many advantages over inhibitor- and tracer-based methods for the determination of production pathways. In addition to δ15Nbulk-N2O and δ18O-N2O, Yoshida and Toyoda (2000) suggested that analyses of the intramolecular distributions of 15N in N2O (Nβ=Nα=O) (isotopomers), which are often expressed as the site preference (SP) (δ15Nα–δ15Nβ), may provide critical information that could help identify the precise sources and sinks of this greenhouse gas. Site preference has emerged as a potential conservative tracer for microbial N2O production because 1) it is independent of the isotopologue composition of the substrates and 2) it does not exhibit changes during the course of production. Distinct site preference values for N2O production due to bacterial denitrification, including nitrifier denitrification, relative to hydroxylamine oxidation and fungal denitrification provide a fundamental basis that facilitates the resolution of production pathways in the natural environment (Sutka et al., 2006, 2008).
Strains of soil ammonia-oxidizing archaea (AOA) in the clade Thaumarchaeota, which were cultivated in laboratories, were shown to be capable of growing as autotrophic ammonia oxidizers (Jung et al., 2011; Lehtovirta-Morley et al., 2011; Kim et al., 2012). The thaumarchaeotal I.1a, I.1a-associated and I.1b clades are frequently recognized as the dominant ammonia-oxidizing organisms in terrestrial environments, possibly because of their high affinity for NH3 (Jung et al., 2011; Kim et al., 2012). Thus, studies on the isotopic signatures of N2O and the pathways of N2O production by AOA are crucial for tracking the sources of N2O emitted from soils and estimating the soil N2O budget. AOA are highly fastidious and hence the ecophysiological characterization of AOA using cultured representatives is largely hindered. Recently, an AOA strain enriched from the ocean was shown to produce N2O with distinct isotopic signatures (Santoro et al., 2011). N2O production by AOA enriched from agricultural soils has been reported (Jung et al., 2011; Kim et al., 2012), but there has been no isotopic analysis of N2O produced by soil AOA. The aim of this study was to investigate the isotopomeric signatures of N2O produced by AOA strains enriched from soils and their pathways of N2O production. This study may provide insights into the N2O production mechanisms of AOA, thereby facilitating the tracking of archaeal sources of N2O in terrestrial environments.
Materials and methods
Cultivation of AOA
Soil AOA strains (Table 1) were enriched from various soils. The enrichment culture procedure was basically the same as that described by Jung et al. (2011) and Kim et al. (2012), and various soil sources were used as inocula (see the Supplementary Materials and Methods). Initial enrichment cultures were set up in allylthiourea (ATU) (20 μM) and chlorate (50 μM) to selectively inhibit the growth of AOB and nitrite oxidizing bacteria (NOB), respectively. The enriched AOA cultures contained heterotrophic bacteria, and attempts were made to further enrich AOA by successive culture in artificial freshwater medium (AFM) containing a mixture of ampicillin and kanamycin (each at 50 μg ml−1). The composition of the culture media is described in the Supplementary Materials and Methods. After ca. 3 years (strains MY1, MY2 and JG1) and 2 years (strains MY3 and CS) of tri-weekly transfer, the cultures were serially diluted (10-fold) to extinction, and the highest dilution showing nitrifying activity was selected for isolating a single archaeal strain.
Soil AOA strains and a reference strain of AOB, Nitrosomonas europaea ATCC 19718, were incubated in an AFM. A marine AOA strain AR used as a reference strain in this study was incubated in an artificial seawater medium (ASM), which was modified from the study by Jung et al. (2013). For the N2O studies, cultures of AOA and a strain of N. europaea were incubated with 50 ml of AFM or ASM in 120 ml serum bottles sealed with a butyl rubber stopper (Bellco Glass, Vineland, NJ, USA). All of the cultures were incubated in the dark at their optimum growth temperatures (Table 1) with unmodified ambient air in the head space. Sampling for gas and chemical analysis and supplementation with chemicals were conducted using syringes. The resistance of the butyl rubber stopper to the leakage of N2O gas for 1 month was confirmed using standard gas. The cultures were routinely supplemented with 1 mM ammonium chloride as the sole energy source. After the ammonia oxidation was completed (in ca three weeks), 5% of the total culture was routinely transferred to fresh medium. The maximum ammonia oxidized by strain CS was ca 0.1 mM and hence strain CS was transferred when no further oxidation of ammonia occurred. The ammonia and nitrite concentrations were determined using an ion chromatograph (ICS-1500, Dionex, Sunnyvale, CA, USA) with an OnGuard II Ag Cartridge (Dionex) and colorimetrically, respectively (Solorzano, 1969). The N2O yield after the completion of ammonia oxidation was determined on the basis of the nitrite produced from the same cultures. Cell growth was determined as described in the Supplementary Materials and Methods.
Isotopic signature of N2O
N2O was analyzed after the ammonia was depleted by oxidation. The ammonia level was monitored by measuring ammonia in the media and was depleted in 3 weeks. Strain CS was analyzed after the oxidation of 0.1 mM ammonium chloride. The headspaces of the 120 ml serum bottles were used for the isotopic N2O analysis. The dual isotope and isotopomer signatures of N2O were analyzed, that is, δ18O of N2O (δ18O-N2O), average δ15N (δ15Nbulk) and δ15N from the central N-position (δ15Nα), after cryo-focusing by isotope ratio mass spectrometry (IRMS) using a Delta V IRMS (Thermo-Fisher, Bremen, Germany), which allowed simultaneous detection of m/z 44, 45 and 46 (Well and Flessa, 2008). The IRMS was connected to a gas chromatograph (GC) (Trace GC Ultra, Thermo-Fisher, Bremen, Germany) and a modified Precon (Thermo-Fisher) equipped with an autosampler (model Combi-PAL, CTC-Analytics, Zwingen, Switzerland) (Well and Flessa, 2008). The 15N SP was determined as follows: δ15Nβ=2 × δ15Nbulk—δ15Nα. The dual isotope and isotopomer ratios of a sample (Rsample) were expressed as the ‰ deviation from 15N/14N and 18O/16O ratios of the reference standard materials (Rstd), atmospheric N2 and standard mean ocean water (SMOW):

where X=15Nbulk, 15Nα, 15Nβ or 18O. The typical analytical precision levels were 0.2, 0.4 and 0.3‰ for δ15Nbulk, δ15Nα and δ18O, respectively.
Isotopic signatures of H2O, NO2−, and NH4+
The δ18O values of the medium water were analyzed using a laser spectrometer (model L 1115-I, Picarro, Santa Clara, CA, USA), which was suitable for analyzing water as a liquid or vapor. To determine δ15N and δ18O values for NO2− in the media, the denitrifier method was used to transform NO2− quantitatively to N2O (Casciotti et al., 2002). The subsequent isotope analysis was conducted as described above. The δ15N value of NH4+ and NO2− was measured by combustion of the pure salts (ammonium chloride and sodium nitrite, Sigma-Aldrich) using an elemental analyzer coupled to an IRMS (DeltaPlus, Thermo-Finnigan, Bremen, Germany).
N2O tracer experiment
The N2O tracer experiment was performed using the same culture conditions used for the isotope signature analysis. However, 0.5 mM 99 atm% 15NH4Cl (Cambridge Isotope laboratories, Tewksbury, MA, USA) was used as the sole electron donor in the presence of excess unlabeled nitrite (2 mM NaNO2), which was added as a background to determine the N2O fractions derived from preexisting nitrite via nitrifier denitrification. The same cultures used in the trace experiment with unlabeled substrates (0.5 mM unlabeled ammonium chloride with 2 mM unlabeled nitrite) were also used for comparative SP analysis. The amount of N2O gas in the headspace was measured using a GC with a quadrupole mass spectrometer (QMS) (6890A (G1540A)/5973N, Agilent, Santa Clara, CA, USA). The quadrupole mass spectrometer recorded the relative quantitative intensity of ions according to their mass-to-charge ratios (m/e). From 15N-labeled NH4+ and unlabeled NO2−, there were three possible combinations of N isotopes for N2O: 14,14N2O (m/e 44), 14,15N2O (m/e 45) and 15,15N2O (m/e 46). The N2O ion peaks produced by electron impact ionization were measured in these three m/e positions and their background in the mass spectrometer was subtracted. Gas samples were taken from the headspace of culture bottles using a 5 ml gas-tight syringe and injected via a gas sampling valve using a 1 ml sample loop in the GC. The GC was fitted with a 4 m stainless steel column packed with Porapak Q (80/100 mesh, Restek, Bellefonte, PA, USA) to separate N2O and CO2 from the sample gas. The oven was isothermal at 50 °C and the N2O ion peaks were measured three times for each sample. We used six certified reference gas mixtures of N2O in nitrogen, that is, 0.198, 0.331, 0.550, 0.798, 12.0, and 94.9 N2O μmol mol−1, to calibrate the GC/QMS. These reference gas mixtures were prepared gravimetrically by the Korea Research Institute for Standards and Science and verified by international comparisons, that is, CCQM-K68 in 2010 (Lee et al., 2011).
Effect of PTIO on nitrification
To evaluate the crucial role of NO as an intermediate in nitrification and N2O production by soil AOA, the effect of 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxide-3-oxyl (100 μM, PTIO), a NO scavenger (Amano and Noda, 1995; Ellis et al., 2001), was tested on the growth and ammonia oxidation activities of AOA. The PTIO experiment was performed using the same growth conditions as those used for the isotopic signature studies. Cell growth and ammonia oxidation were determined during and after incubation.
Results and discussion
AOA strains
The soil AOA strains used in this study are shown in Table 1. An AOA strain (AR) from a marine sediment (Park et al., 2010) and an AOB strain were also included as references. The phylogenetic analysis performed using archaeal 16S rRNA and amoA genes as described in Supplementary Materials and Methods indicated that I.1a (MY1, MY2 and AR), I.1a-associated (CS) and I.1b (MY3 and JG1) clades within Thaumarchaeota were included (Figure 1). The phylogenetic information regarding enriched AOA strains (based on 16S rRNA and amoA gene sequences) is described in the Supplementary Results.
Comparative phylogenetic analysis of (a) 16S rRNA and (b) amoA gene sequences from Thaumarchaeota strains used in this study. The classification of thaumarchaeotal groups is based on Ochsenreiter et al. (2003). Branching patterns supported by bootstrap values (1000 iterations) >50% according to the neighbor-joining method are denoted by their bootstrap values. Strains indicated in red were used in this study. Strains marked in bold are reference strains or fosmid clones of Thaumarchaeota. The origins of each clone or strain are indicated in parentheses with their accession numbers. The number of 16S rRNA and amoA gene sequences used for checking the purity of AOA cultures is indicated in the second set of parentheses.
All five soil AOA cultures used in this study grew on minimal medium, with ammonia as the sole electron donor. Each AOA culture was highly enriched and contained a single archaeal strain, as revealed by a single band corresponding to that of archaeal 16S rRNA gene sequence in a denaturing gradient gel electrophoresis. As described previously, clone libraries derived from the enrichment cultures confirmed that only one unique sequence (>99.7% identity within the error rate expected for PCR amplification (von Wintzingerode et al., 1997; Könneke et al., 2005)) for the archaeal 16S rRNA and amoA genes, respectively, was identified in each culture (Jung et al., 2011; Kim et al., 2012). The number of clones (>37 per library) that were sequenced and analyzed is shown in Figure 1. FISH with domain-specific probes and real-time quantitative PCR analysis confirmed that the archaeal abundance was >90% of the minor bacterial cells co-cultured. In this study, AOB were selectively inhibited at 20 μM ATU as reported previously (Bedard and Knowles, 1989; Ginestet et al., 1998; Jung et al., 2011). As reported by Santoro et al. (2011) and Shen et al. (2013), the AOA used in this study were not inhibited at 20 μM ATU (apart from strain CS, which was partially inhibited at 20 μM ATU, as previously reported in Lehtovirta-Morley et al. (2013)). The absence of AOB contamination was confirmed by the inability to amplify the bacterial 16S rRNA gene from betaproteobacterial AOB using specific nested PCR primers, and the amoA genes from gamma and betaproteobacterial AOB using specific PCR primers (Supplementary Table S1). The PCR results were also supported by the analysis of bacterial 16S rRNA gene clone libraries (Supplementary Table S2). Only the archaeal growth was tightly coupled to ammonia oxidation, as shown in other studies (Jung et al., 2011; Kim et al., 2012). These results suggested that ammonia-oxidizing archaea were solely responsible for the observed ammonia oxidation.
N2O production by AOA
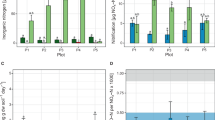
We observed N2O production in all AOA cultures where ammonia oxidation occurred (Table 1). The inocula (early stationary phase cultures) had initial cell densities of ca 1 × 106 to 5 × 106 16S rRNA gene copies ml−1 for all AOA cultures and N. europaea. The cultures of AOA had stationary phase cell densities of ca 5 × 107 to 2 × 108 16S rRNA gene copies ml−1 after the oxidation of 1 mM ammonia, except strain CS (Table 1). The N2O production by the AOA strains ranged from 0.041 to 0.419 μmol (N2O-N) after the exhaustion of 1 mM ammonia. The yields (N2O/NO2− produced) from AOA ranged from 0.11 to 4.28%, which were comparable to those from N. europaea (Table 1) and other AOB (Colliver and Stephenson, 2000). The total amount and production yield of N2O from strains JG1 and CS were at the upper end of the range. The highest N2O production yield from the acidophilic strain CS might have been related to its high sensitivity to nitrite toxicity (Supplementary Figure S1). Indeed, ca 0.1 mM nitrite was the maximum tolerable concentration for the CS cultures, as found with Nitrosotalea devanaterra (Lehtovirta-Morley et al., 2011).
The archaeal strains were not pure isolates, but the involvement of minor contaminant (denitrifying) bacteria with N2O production could be rejected based on the following experimental evidence. (1) N2O production and ammonia oxidation were tightly coupled and N2O production ceased after ammonia oxidation was completed, as found using strain MY1 (Jung et al., 2011) and JG1 (Kim et al., 2012) (see Supplementary Figure S2). This indicated that the contribution of bacterial denitrification to N2O production from the nitrite and organic debris produced by AOA was negligible. (2) Chlorite (50 μM; ClO2−), an ammonia oxidation inhibitor that does not inhibit bacterial denitrification, completely inhibited N2O production in the presence of 1 mM nitrite (Jung et al., 2011; Kim et al., 2012). Even when yeast extract (10 mg l−1) was spiked in the medium containing chlorite, N2O production was still not observed in the presence of 1 mM nitrite (data not shown). In this condition, the only bacterial cell growth (up to 108 cells ml−1) that occurred corresponded to the final bacterial cell concentrations in the ammonia-oxidizing enrichment culture. (3) Heterotrophic bacterial strains with a denitrification capability (Pseudomonas sp. from culture JG1; Acinetobacter sp. from culture MY1; Rhodococcus sp. from culture MY3) were isolated from the ammonia-oxidizing cultures and cultivated with 10 mg l−1 yeast extract and 0.5 mM nitrite using the same medium and incubation conditions. The amount of N2O produced after 2 weeks was negligible and was close to the atmospheric background level (0.003–0.005 N2O-N μmol) (Supplementary Figure S3). These results showed clearly that the cultivation conditions were sufficiently oxic that N2O production from bacterial denitrification was negligible.
Isotopic signatures of N2O
In addition to bulk isotopic information (δ15N-N2O and δ18O-N2O), the N2O molecule contains position-dependent isotopic information from nitrogen (δ15Nα and δ15Nβ). SP, the position-dependent isotope value of N2O, is an isotopic signature that is commonly used to interpret the source of N2O production (Toyoda and Yoshida, 1999; Sutka et al., 2003; Ostrom and Ostrom, 2011). Studies using pure AOB cultures have indicated that a positive SP (30–38‰) is consistent with a N2O source from hydroxylamine oxidation (ammonia oxidation), whereas nitrifier denitrification (and denitrification) is thought to produce N2O with a SP value near or below zero (–10 to 0‰) (Frame and Casciotti, 2010; Sutka et al., 2006). The N2O emissions from various soils have positive (0–35‰) SP signatures (Pérez et al., 2001; Park et al., 2011, 2012; Toyoda et al., 2011), which indicates that ammonia oxidation is an important source of soil N2O. The SP values of soil AOA strains (MY1-3 and JG1) were distinct and they ranged from 20 to –30‰, except strain CS (Table 2). The SP values of strain AR and N. europaea were at the upper end of the range. Intriguingly, the SP value of strain CS was 13‰, which was much lower than other ammonia-oxidizing microorganisms. Nitrite toxicity increased the nitrifier denitrification activity required for detoxification, which might have been attributable to the low SP and the high N2O production by strain CS (Tables 1 and 2). A future SP analysis of AOB at acidic pH might be useful to verify whether the low SP value can be attributed to the increase in nitrifier denitrification required for nitrite detoxification under acidic conditions. The results of the present study indicated that SP analysis might not be a definitive method for distinguishing archaeal and bacterial nitrification sources of N2O production.
The δ15Nbulk-N2O values for all AOA strains were –13 to –17‰, except strain CS (–35.54‰), whereas the value for N. europaea was –19.88‰ (Table 2). The end-member mixing model was not applicable to the CS culture because of the partial oxidation of ammonia and hence the 15Nbulk-N2O produced by strain CS did not reflect the isotopic shifts of NH4+. The ranges of δ15Nbulk-N2O from soil AOA were similar to those (–10 to –50‰) from agricultural soils and forest soils (Kim and Craig, 1993; Rahn and Wahlen, 2000; Pérez et al., 2001; Rock et al., 2007). Previous studies found that the δ15Nbulk-N2O values of marine AOB (Nitrosomonas marina C-113) were about –10‰ with 20% O2 concentration (Frame and Casciotti, 2010), whereas the δ15Nbulk-N2O values of N. europaea (ATCC 19718) in ammonia-oxidizing and nitrite-reducing conditions were –46.5‰ and –34.0‰, respectively (Sutka et al., 2003, 2006). Surprisingly, the δ15Nbulk-N2O values of a marine AOA strain CN25 were positive (8.7‰) (Santoro et al., 2011), which differed significantly from the ideal isotope fractionation effect and from those of the soil AOA strains. These values were similar to those from surface oceans and the troposphere (5‰ to 10‰) (Kim and Craig, 1993; Rahn and Wahlen, 2000; Popp et al., 2002). Strain AR was also from a marine environment but its δ15Nbulk value (–12.91‰) was similar to those of the soil AOA strains and marine AOB (Frame and Casciotti, 2010). The ‘apparent’ (observable) isotope effect calculated from the data obtained by Santoro et al. (2012) would be  =δ15Nbulk-N2O−δ15N-NH4=8.7−2.5=6.2‰. As the intrinsic isotope effects of N2O formation are always negative, that is, δ15N of product is depleted when compared with δ15N of substrate, our value of
=δ15Nbulk-N2O−δ15N-NH4=8.7−2.5=6.2‰. As the intrinsic isotope effects of N2O formation are always negative, that is, δ15N of product is depleted when compared with δ15N of substrate, our value of  =δ15Nbulk-N2O−δ15N-NH4=−17−(−1)=−16‰ is much closer to the expected value. The deviation of the apparent isotope effect from the intrinsic isotope effect (ideal Rayleigh behavior) could be caused by variations in the N2O yield of nitrification over time. For example, if (1) the N2O yield is higher at the end when the residual substrate (NH4+) is already substantially δ15N-enriched, or if (2) clumps of cells in the culture media create local areas of δ15N-enriched NH4+, the apparent isotope effect could be reversed (that is, positive).
=δ15Nbulk-N2O−δ15N-NH4=−17−(−1)=−16‰ is much closer to the expected value. The deviation of the apparent isotope effect from the intrinsic isotope effect (ideal Rayleigh behavior) could be caused by variations in the N2O yield of nitrification over time. For example, if (1) the N2O yield is higher at the end when the residual substrate (NH4+) is already substantially δ15N-enriched, or if (2) clumps of cells in the culture media create local areas of δ15N-enriched NH4+, the apparent isotope effect could be reversed (that is, positive).
Different N2O production pathways incorporate elemental O from isotopically distinct sources (for example, H2O or O2) and they have unique fractionation factors (Ostrom et al., 2000). The δ18O-N2O values of surface oceans and the troposphere were 20–40‰, whereas those of agricultural soils and forest soils were 0–60‰ (Kim and Craig, 1993; Pérez et al., 2001; Rock et al., 2007). The δ18O-N2O values of the AOA strains were 29–34‰ for MY1, 2, and 3, ca 22‰ for CS and JG1 and 36‰ for AR. The δ18O-N2O value of N. europaea was 27‰. The oxygen isotope branching effects that affect δ18O enrichment from oxygen sources during N2O production may also be applicable to soil AOA strains as suggested for AOB and marine AOA (Frame and Casciotti, 2010; Santoro et al., 2011). The δ18O-N2O signature was not significantly different from those of AOB and hence the ratio of oxygen atom sources (H2O to O2) incorporated into nitrite (1:1) appears to hold for AOA and AOB. The δ18O-N2O values of marine AOA (strain CN25) were also similar to those of soil AOA. The shift in δ18O-N2O reflected changes in the proportion of N2O derived from two different pathways, that is, ammonia oxidation and nitrifier denitrification; hence, the covariance of SP and δ18O-N2O was observed in AOB. The covariance of SP and δ18O-N2O for soil AOA may be inferred from the plot of SP versus δ18O-N2O, including other ammonia-oxidizing AOA and AOB, as shown in Figure 2. Although the proportion of nitrifier denitrification varied between replicates (Supplementary Table 3 and Supplementary Figure S4), there was a distinct covariance between SP and δ18O-N2O for each strain in the incubation conditions used in this study. This indicated that combined isotopic signatures can be used to improve the process identification, whereas individual signatures had limitations when trying to identify sources (Snider et al., 2012).
Covariance of SP and δ18O-N2O. Symbols shown in red (soil AOA), blue (marine AOA strain AR), and pink (N. europaea) correspond to the data from this study (circles indicate values from ammonia-oxidizing cultures without nitrite addition; triangles indicate values from ammonia-oxidizing cultures with 2 mM nitrite): A, MY3; B, MY1; C, MY2; D, JG1; E, CS; F, MY1 with 2 mM nitrite. The green circle indicates marine AOA strain CN25 (Santoro et al., 2011). The black circles and triangles indicate N. europaea under hydroxylamine-oxidizing and denitrifying conditions, respectively, whereas the gray circle indicates N. europaea under ammonia-oxidizing conditions (Sutka et al., 2003; Sutka et al., 2006). The error bars are based on replicate experiments. The raw data used in this plot are presented in Supplementary Table S3.
There were similar covariance results from N2O reduction to N2 during denitrification (Jinuntuya-Nortman et al., 2008; Well and Flessa, 2009). The SP/δ18O-N2O slope of N2O emitted from soil AOA (approx. 1) (Figure 2) was within the slope range reported for residual N2O after partial reduction to N2 (Well et al., 2012). This similarity might further complicate the distinction of N2O from nitrification and denitrification in environments.
15N-labeling experiments
The mixing of two different N2O production pathways by AOB (that is, ammonia oxidation and nitrifier denitrification) results in different SP values, which are well established using various approaches. The SP signatures of N2O can be used to calculate the magnitude of each pathway’s contribution to the total N2O output based on its SP signature for AOB (Sutka et al., 2003; Sutka et al., 2006).
The mass of N2O produced during the oxidation of 15N-labeled NH4+ (0.5 mM) in the presence of excess unlabeled NO2− (2 mM) was determined to trace the sources of N2O. The tracer experiment with N. europaea (Ritchie and Nicholas, 1972) suggested that the dimerization of 15NOH, derived from 15NH2OH, and 14NOH, derived from nitrite, might subsequently lead to the formation of 14,15N2O. Three possible combinations of the N isotopes in N2O were also possible for soil AOA via two different processes: 14,14N2O (nitrifier denitrification, m/e 44), 14,15N2O (mixing of nitrifier denitrification and ammonia oxidation, m/e 45) and 15,15N2O (ammonia oxidation, m/e 46) (Figure 3a). Strain CS could not be included in this experiment because of its high sensitivity to NO2−. The N2O mass ratios varied, depending on the strains (Figure 3b). N2O produced by the mixed process was dominant in JG1 and N. europaea, where the three different mass ratios of N2O (m/e 46:45:44) from JG1 and N. europaea were 20.4%:71.2%:8.4% and 28.8%:62.8%:8.4%, respectively. Most of the N2O produced by strains MY1, MY2, MY3 and AR had m/e values of 45 and 44, which indicated that N2O derived from the mixed process and nitrifier denitrification were co-dominant (up to 90%) (Figure 3b). The proportions of mixed and pure ammonia-derived N2O were not correlated with the SP values shown in Table 2. For example, SP was not high in strain JG1 despite the high proportion of mixed and pure ammonia-derived N2O. The mixing process might be a key step that contributes to the high SP of ammonia-derived N2O. The contribution made by nitrifier denitrification to N2O production might be overestimated due to the high background (2 mM) nitrite level. In the present study, we observed an increased N2O yield and reduced SP values in strain MY1 and in N. europaea in the presence of 2 mM nitrite (Figure 2, Table 2, and Supplementary Table S3), which indicated enhanced nitrifier denitrification; similar findings were reported for AOB (Frame and Casciotti, 2010). Therefore, the contribution made by the direct conversion of 15N-labeled NH4+ to N2O production, that is, via ammonia oxidation (m/e 46) and the mixed process (m/e 45), might be more significant in the absence of background nitrite. Thus, the tracer experiment was not applicable for determining the exact contributions made by the different processes in the isotopomer study. Furthermore, the fraction of N2O produced via nitrifier denitrification by AOA in soil might be much lower because of the presence of nitrite-oxidizing bacteria. This study indicated that the isotopic signatures of N2O produced by AOA might have been due to combinations of two different N2O production processes (see Figure 3), similar to AOB (Ritchie and Nicholas, 1972). In a similar experiment using marine AOA with 15N-labeled NH4+ addition, at least some of the N2O was shown to be produced directly from the 15N-enriched NH3 pool without passing through the nitrite pool (Santoro et al., 2011).
(a) Proposed biochemical processes during archaeal nitrification, showing the pathways leading to N2O production in the tracer experiment. Two different NO sources permitted three possible combinations for N2O production. HNO (nitroxyl) or hydroxylamine has been proposed as an intermediate during the oxidation of ammonia to nitrite (Walker et al., 2010). (b) The composition of labeled N2O produced during the tracer experiment. Soil AOA strains were incubated in the presence of 0.5 mM 15N-labeled NH4+ and 2 mM nitrite.
Effect of PTIO on nitrification
Bacterial ammonia oxidation is traditionally defined as the oxidation of ammonia to nitrite via hydroxylamine as an intermediate. In addition to hydroxylamine, the reactive intermediate nitroxyl (nitroxyl hydride; HNO) has been proposed as a key intermediate during archaeal ammonia oxidation (Walker et al., 2010). The present study showed that archaeal ammonia oxidation was very sensitive to PTIO, a scavenger of free radical nitric oxide (NO) and nitroxyl (Amano and Noda, 1995; Ellis et al., 2001; Samuni et al., 2010), and neither ammonia oxidation nor archaeal growth was observed at 100 μM PTIO (Supplementary Figure S5, and Yan et al. (2012)). In addition, the inhibitory effects of 100 μM PTIO on AOB were negligible (Supplementary Figure S5 and Shen et al. (2013)). Recently, Vajrala et al. (2013) demonstrated hydroxylamine-induced oxygen consumption and ATP production by the marine AOA strain, Nitrosopumilus maritimus. In this case, NO was suggested to act as an electron shuttling factor in the same way as the quinone moiety in AOB. High expression of nirK gene was observed in strain MY1 during ammonia oxidation (Jung et al., 2011). A more plausible explanation may be that the inhibitory effect of PTIO is related to NO-based electron shuttling in the hydroxylamine pathway rather than to the production of nitroxyl intermediates. Our results suggest that the ammonia oxidation mechanism in AOA and the N2O production mechanism in AOA via ammonia oxidation might be distinct from those of AOB. By contrast, the isotopic signatures of δ15N-N2O and δ18O-N2O, the SP, and the results of the labeled tracer experiment did not differ greatly from those of the AOB strains.
Conclusions
Soil is the most important source of global N2O emissions, and a significant but unknown fraction of the soil N2O is produced by nitrification. The recent discovery of AOA in soils implies that it is essential to revise the current assumption that AOB are the sole sources of N2O from soil nitrification. The present study showed that the isotopic signatures (SP, δ15Nbulk-N2O, and δ18O-N2O) of soil AOA strains were similar to those of AOB strains and also similar to those from agricultural soils and forest soils. Thus, the activities and roles of soil AOA during N2O emission from soil have been hidden in previous isotopic studies of N2O. The current study, based on soil AOA cultures, indicates that a significant amount of N2O could be produced from archaeal nitrification. However, there is controversy regarding AOA’s contribution to autotrophic nitrification in terrestrial systems, despite their numerical dominance over AOB (Schleper, 2010). Global climate change and the upturn in atmospheric N2O imply that it is imperative to incorporate archaeal N2O production processes into global N2O models to accurately predict the response of the N2O flux in future soils. Selective inhibitors of nitrification, such as ATU for AOB and PTIO for AOA, could provide an important tool for differentiating the activity of soil AOA from that of AOB with respect to N2O emissions. These results may provide fundamental information for facilitating future studies on the N2O production pathway in AOA and the tracking of N2O emission sources in soils.
References
Amano F, Noda T . (1995). Improved detection of nitric oxide radical (NO•) production in an activated macrophage culture with a radical scavenger, carboxy PTIO and Griess reagent. FEBS Lett 368: 425–428.
Arp DJ, Stein LY . (2003). Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol 38: 471–495.
Bedard C, Knowles R . (1989). Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53: 68–84.
Casciotti KL, Sigman DM, Hastings MG, Bohlke JK, Hilkert A . (2002). Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74: 4905–4912.
Colliver BB, Stephenson T . (2000). Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol Adv 18: 219–232.
Conrad R . (1996). Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60: 609–640.
Ellis A, Lu H, Li CG, Rand MJ . (2001). Effects of agents that inactivate free radical NO (NO•) on nitroxyl anion-mediated relaxations, and on the detection of NO• released from the nitroxyl anion donor Angeli's salt. Br J Clin Pharmacol 134: 521–528.
Frame CH, Casciotti KL . (2010). Biogeochemical controls and isotopic signatures of nitrous oxide production by a marine ammonia-oxidizing bacterium. Biogeosciences 7: 2695–2709.
Gödde M, Conrad R . (1999). Immediate and adaptational temperature effects on nitric oxide production and nitrous oxide release from nitrification and denitrification in two soils. Biol Fertil Soils 30: 33–40.
Ginestet P, Audic JM, Urbain VV, Block JC . (1998). Estimation of nitrifying bacterial activities by measuring oxygen uptake in the presence of the metabolic inhibitors allylthiourea and azide. Appl Environ Microbiol 64: 2266–2268.
Hendriks J, Oubrie A, Castresana J, Urbani A, Gemeinhardt S, Saraste M . (2000). Nitric oxide reductases in bacteria. Biochim Biophys Acta 1459: 266–273.
Jinuntuya-Nortman M, Sutka RL, Ostrom PH, Gandhi H, Ostrom NE . (2008). Isotopologue fractionation during microbial reduction of N2O within soil mesocosms as a function of water-filled pore space. Soil Biol Biochem 40: 2273–2280.
Jung MY, Park SJ, Min D, Kim JS, Rijpstra WI, Sinninghe Damste JS et al. (2011). Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol 77: 8635–8647.
Jung MY, Shin KS, Kim S, Kim SJ, Park SJ, Kim JG et al. (2013). Hoeflea halophila sp. nov., a novel bacterium isolated from marine sediment of the East Sea, Korea. Antonie Van Leeuwenhoek 103: 971–978.
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA . (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546.
Kim JG, Jung MY, Park SJ, Rijpstra WI, Sinninghe Damste JS, Madsen EL et al. (2012). Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14: 1528–1543.
Kim KR, Craig H . (1993). Nitrogen-15 and oxygen-18 characteristics of nitrous oxide: a global perspective. Science 262: 1855–1857.
Lee JB, Lee JS, Moon DM, Kim JS, Wessel R, Aoki N et al. (2011). Final report on international comparison CCQM-K68: nitrous oxide in synthetic air. Metrologia 48: 08004.
Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW . (2011). Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA 108: 15892–15897.
Lehtovirta-Morley LE, Verhamme DT, Nicol GW, Prosser JI . (2013). Effect of nitrification inhibitors on the growth and activity of Nitrosotalea devanaterra in culture and soil. Soil Biol Biochem 62: 129–133.
Mariotti A, Germon JC, Hubert P, Kaiser P, Letolle R, Tardieux A et al. (1981). Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant and Soil 62: 413–430.
Nevison C, Holland E . (1997). A reexamination of the impact of anthropogenically fixed nitrogen on atmospheric N2O and the stratospheric O3 layer. J Geophys Res 102: 25519–25536.
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C . (2003). Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5: 787–797.
Ostrom N, Ostrom P . (2011). The isotopomers of nitrous oxide: analytical considerations and application to resolution of microbial production pathways. In: Baskaran M (ed). Handbook Environ Isot Geochem. Springer: Berlin Heidelberg, pp 453–476.
Ostrom NE, Russ ME, Popp B, Rust TM, Karl DM . (2000). Mechanisms of nitrous oxide production in the subtropical North Pacific based on determinations of the isotopic abundances of nitrous oxide and di-oxygen. Chemosphere—Glob Change Sci 2: 281–290.
Pérez T, Trumbore SE, Tyler SC, Matson PA, Ortiz-Monasterio I, Rahn T et al. (2001). Identifying the agricultural imprint on the global N2O budget using stable isotopes. J Geophys Res 106: 9869–9878.
Park BJ, Park SJ, Yoon DN, Schouten S, Sinninghe Damsté JS, Rhee SK . (2010). Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in co-culture with sulfur-oxidizing bacteria. Appl Environ Microbiol 76: 7575–7587.
Park S, Pérez T, Boering KA, Trumbore SE, Gil J, Marquina S et al. (2011). Can N2O stable isotopes and isotopomers be useful tools to characterize sources and microbial pathways of N2O production and consumption in tropical soils? Glob Biogeochem Cycle 25: GB1001.
Park S, Croteau P, Boering KA, Etheridge DM, Ferretti D, Fraser PJ et al. (2012). Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat Geosci 5: 261–265.
Popp BN, Westley MB, Toyoda S, Miwa T, Dore JE, Yoshida N et al. (2002). Nitrogen and oxygen isotopomeric constraints on the origins and sea-to-air flux of N2O in the oligotrophic subtropical North Pacific gyre. Glob Biogeochem Cycle 16: 1064.
Rahn T, Wahlen M . (1997). Stable isotope enrichment in stratospheric nitrous oxide. Science 278: 1776–1778.
Rahn T, Wahlen M . (2000). A reassessment of the global isotopic budget of atmospheric nitrous oxide. Glob Biogeochem Cycle 14: 537–543.
Ritchie GA, Nicholas DJ . (1972). Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J 126: 1181–1191.
Rock L, Ellert BH, Mayer B, Norman AL . (2007). Isotopic composition of tropospheric and soil N2O from successive depths of agricultural plots with contrasting crops and nitrogen amendments. J Geophys Res 112: D18303.
Samuni U, Samuni Y, Goldstein S . (2010). On the distinction between nitroxyl and nitric oxide using nitronyl nitroxides. J Am Chem Soc 132: 8428–8432.
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL . (2011). Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333: 1282–1285.
Schleper C . (2010). Ammonia oxidation: different niches for bacteria and archaea? ISME J 4: 1092–1094.
Seitzinger SP, Kroeze C, Styles RV . (2000). Global distribution of N2O emissions from aquatic systems: natural emissions and anthropogenic effects. Chemosphere—Global Change Sci 2: 267–279.
Shen T, Stieglmeier M, Dai J, Urich T, Schleper C . (2013). Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344: 121–129.
Snider DM, Venkiteswaran JJ, Schiff SL, Spoelstra J . (2012). Deciphering the oxygen isotope composition of nitrous oxide produced by nitrification. Glob Change Biol 18: 356–370.
Solorzano L . (1969). Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14: 799–801.
Sutka RL, Ostrom NE, Ostrom PH, Gandhi H, Breznak JA . (2003). Nitrogen isotopomer site preference of N2O produced by Nitrosomonas europaea and Methylococcus capsulatus Bath. Rapid Commun Mass Spectrom 17: 738–745.
Sutka RL, Ostrom NE, Ostrom PH, Breznak JA, Gandhi H, Pitt AJ et al. (2006). Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl Environ Microbiol 72: 638–644.
Sutka RL, Adams GC, Ostrom NE, Ostrom PH . (2008). Isotopologue fractionation during N2O production by fungal denitrification. Rapid Commun Mass Spectrom 22: 3989–3996.
Toyoda S, Yoshida N . (1999). Determination of nitrogen isotopomers of nitrous oxide on a modified isotope ratio mass spectrometer. Anal Chem 71: 4711–4718.
Toyoda S, Yano M, Nishimura S-i, Akiyama H, Hayakawa A, Koba K et al. (2011). Characterization and production and consumption processes of N2O emitted from temperate agricultural soils determined via isotopomer ratio analysis. Glob Biogeochem Cycle 25: GB2008.
Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA et al. (2013). Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA 110: 1006–1011.
von Wintzingerode F, Gobel UB, Stackebrandt E . (1997). Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21: 213–229.
Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107: 8818–8823.
Well R, Flessa H . (2008). Isotope fractionation factors of N2O diffusion. Rapid Commun Mass Spectrom 22: 2621–2628.
Well R, Flessa H . (2009). Isotopologue enrichment factors of N2O reduction in soils. Rapid Commun Mass Spectrom 23: 2996–3002.
Well R, Eschenbach W, Flessa H, von der Heide C, Weymann D . (2012). Are dual isotope and isotopomer ratios of N2O useful indicators for N2O turnover during denitrification in nitrate-contaminated aquifers? Geochim Cosmochim Acta 90: 265–282.
Wrage N, Velthof GL, van Beusichem ML, Oenema O . (2001). Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33: 1723–1732.
Yan J, Haaijer SC, Op den Camp HJ, van Niftrik L, Stahl DA, Konneke M et al. (2012). Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory-scale model system. Environ Microbiol 14: 3146–3158.
Yoshida N, Toyoda S . (2000). Constraining the atmospheric N2O budget from intramolecular site preference in N2O isotopomers. Nature 405: 330–334.
Acknowledgements
We thank Dr Jin-Seog Kim (Korea Research Institute of Standards and Science) for assistance with N2O measurements. This work was supported by the Basic Science Research Program (2012R1A1A2A10039384), the Mid-Career Researcher Program (NRF-2013R1A2A2A05006754) through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, the Korea Polar Research Institute (PP10040), and the Marine and Extreme Genome Research Center Program of the Ministry of Land, Transportation and Maritime Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Jung, MY., Well, R., Min, D. et al. Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J 8, 1115–1125 (2014). https://doi.org/10.1038/ismej.2013.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2013.205
Keywords
This article is cited by
-
The contributions of ammonia oxidizing bacteria and archaea to nitrification-dependent N2O emission in alkaline and neutral purple soils
Scientific Reports (2022)
-
The Effects of N Enrichment on Microbial Cycling of Non-CO2 Greenhouse Gases in Soils—a Review and a Meta-analysis
Microbial Ecology (2022)
-
The contribution of nirK gene-containing thaumarchaea to denitrification and N2O production across coastal sediment and terrestrial ecosystems
Journal of Soils and Sediments (2022)
-
Soil gas probes for monitoring trace gas messengers of microbial activity
Scientific Reports (2021)
-
Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems
Reviews in Environmental Science and Bio/Technology (2020)