Abstract
Disturbances act as powerful structuring forces on ecosystems. To ask whether environmental microbial communities have capacity to recover after a large disturbance event, we conducted a whole-ecosystem manipulation, during which we imposed an intense disturbance on freshwater microbial communities by artificially mixing a temperate lake during peak summer thermal stratification. We employed environmental sensors and water chemistry analyses to evaluate the physical and chemical responses of the lake, and bar-coded 16S ribosomal RNA gene pyrosequencing and automated ribosomal intergenic spacer analysis (ARISA) to assess the bacterial community responses. The artificial mixing increased mean lake temperature from 14 to 20 °C for seven weeks after mixing ended, and exposed the microorganisms to very different environmental conditions, including increased hypolimnion oxygen and increased epilimnion carbon dioxide concentrations. Though overall ecosystem conditions remained altered (with hypolimnion temperatures elevated from 6 to 20 °C), bacterial communities returned to their pre-manipulation state as some environmental conditions, such as oxygen concentration, recovered. Recovery to pre-disturbance community composition and diversity was observed within 7 (epilimnion) and 11 (hypolimnion) days after mixing. Our results suggest that some microbial communities have capacity to recover after a major disturbance.
Similar content being viewed by others
Introduction
Disturbances, defined as events that trigger a discrete change in the physical or chemical environment that may affect the local community (Glasby and Underwood, 1996), have a key role in the structuring of ecosystems (Sousa, 1984; White and Pickett, 1985). Characteristics and ecological consequences of disturbances vary immensely (Fraterrigo and Rusak, 2008), hindering advances in the theoretical and predictive models (Sousa, 1984; White and Jentsch, 2001). As there is an inverse relationship between disturbance frequency and magnitude (large events seldom occur, small events occur frequently (Sousa, 1984; Romme et al., 1998; Turner et al., 1998; Turner and Dale, 1998; White and Jentsch, 2001), extreme disturbances are inherently difficult to study in nature. Nonetheless, observations after large disturbances are crucial for understanding the susceptibility of ecosystems to extreme events such as those expected under altered climate scenarios (for example, Katz and Brown (1992) and works cited).
Microbial communities are at the heart of all ecosystem functions, and thus their responses to disturbances may influence ecosystem recovery (Allison and Martiny, 2008). Some have advised that microbial variables be included in predictive models of ecosystem change (for example, Arhonditsis and Brett, 2004; McGuire and Treseder, 2010; Sarmento et al., 2010). But, before this can occur, it is necessary to understand the robustness of microbial assemblages to disturbances (Allison and Martiny, 2008). In ecology, community robustness is comprised of resistance, defined as the ability to withstand change in the face of a disturbance, and resilience, the pace of recovery (if any) following a disturbance (Pimm, 1984).
Water column mixing is a disturbance to microbial communities because it disrupts the physical–chemical gradients created by thermal stratification known to define niches for microorganisms (for example, Heaney and Talling, 1980; Vincent et al., 1984; Ovreas et al., 1997; Cytryn et al., 2000; Fenchel and Finlay, 2008). From an ecological perspective, bacterioplankton responses to mixing may provide unique insight into understanding disturbances. As it disrupts gradients in the water column and occurs seasonally, mixing affects spatial and temporal drivers of microbial communities. Further, both microbial and environmental responses to mixing are tractable. High-throughput sequencing and fingerprinting methods can be used to assess changes in microbial community diversity, while environmental sensors can be used to quantify spatial and temporal changes in the environment. The alignment of these measurements allows a comprehensive perspective of microbial responses to an ecosystem-level disturbance. Therefore, lake mixing and microbial communities together create an interesting model system for understanding disturbance ecology.
The work described here builds on a series of studies focused on the response of freshwater microbial communities to mixing (Shade et al., 2007, 2008, 2010a, 2010b, 2011; Jones et al., 2008). In an earlier study, we observed that patterns of bacterial community succession in a temperate eutrophic lake were linked to spring and fall mixing events (Shade et al., 2007), suggesting an importance of lake mixing for community dynamics. In a second study of a small, darkly stained sub-tropical lake, we observed a surprising recovery among bacterial communities following mixing events caused by typhoons, with repeatable trajectories of community change across typhoon events and across years (Jones et al., 2008; Shade et al., 2010b). The results of these two surveys prompted us to ask whether bacteria in temperate lakes that mix seasonally would be similarly resilient to an episodic mixing event. Therefore, we conducted an enclosure experiment in a temperate humic lake to evaluate the effect of mixing and specific mixing-associated environmental changes on communities in the epilimnion (near surface stratum) and hypolimnion (bottom stratum) (Shade et al., 2011). Both epilimnion and hypolimnion communities returned to their control composition within 10 days, demonstrating community robustness despite differences in initial compositions and in disturbance characteristics. Finally, we conducted a reciprocal transplant experiment to ask how epilimnion and hypolimnion bacterial communities responded to environmental conditions in the opposite strata (Shade et al., 2010a). Consequently, we identified possible ‘generalist’ community members, persistent in both epilimnion and hypolimnion, that could serve as pioneer species after mixing. The results from the transplant experiment suggested that lake bacterial communities harbor members that initiate post-disturbance succession and eventual recovery.
Following from the results of this series of studies, we asked whether freshwater bacterial communities would be robust (resistant and resilient) to an artificial mixing event that occurred during summer stratification, which is a time when communities are compositionally distinct from communities that occur during seasonal spring and fall mixing events. Mixing at the peak of summer stratification is a disturbance that would not happen naturally, and therefore represents an extreme event for summer microbial communities. Thus, this disturbance experiment provided a unique opportunity to measure both community and ecosystem responses to a large, unlikely disturbance in a relatively well-studied system. Summer mixing also allowed us to observe changes in the bacterial community separate from other seasonal changes that are concurrent with spring and autumn mixing (for example, ice-off). Further, a whole-ecosystem experiment avoided limitations of mesocosm and enclosure experiments (Schindler, 1998). One common criticism of mesocosms is that they fail to reproduce conditions in nature and provide limited insight, and so it was possible that the community responses we previously observed in mesocosm experiments might not occur in the whole-lake. For these reasons, we wanted to pursue a whole-ecosystem experiment to understand microbial community responses to an unlikely, extreme mixing event.
Here we report the bacterial community responses to the disturbance of an entire small temperate lake, North Sparkling Bog (Boulder Junction, WI, USA, Supplementary Table 1). We assessed bacterioplankton communities using 16S ribosomal RNA gene tag pyrosequencing to gain an unprecedented depth of sampling of the lake microbial diversity.
Materials and Methods
Experiment, site, environmental sensors and sampling
The study lake, North Sparkling Bog (46°00'17.47’ N, 89°42'18.37’ W), is darkly stained with high dissolved organic carbon (average 9.5 mg l−1). Though this is a relatively shallow lake (4.5 m maximum depth), the darkly stained color and surface area (0.46 ha) to depth ratio (1022 m) causes strong stratification during most of the ice-free season, which creates strong vertical gradients in temperature and dissolved oxygen, and overall high stability. North Sparkling Bog is a Global Lakes Ecological Observatory Network site. It has a buoy instrumented with environmental sensors (see below); sensor data are available online (www.gleon.org) and lake characteristics are given in Supplemental Table 1. Whole-lake destratification was performed by mixing epilimnion and hypolimnion waters with the aid of buoyant forces (Read et al., 2011). Briefly, two rubber membranes with inflatable tubing on the outer rim (8.25 m diameter) were oscillated in the water column. When the tubing was filled with compressed air, the membranes rose to the lake surface. After air was released from the tubing, the membrane sank to the bottom. This repeated process of inflation and deflation caused turbulence and internal waves, and eventually resulted in complete mixing.
The lake was stratified in oxygen and temperature before the artificial mixing that began on 02 July 2008. An autoprofiling sonde (Yellow Springs Instruments, Yellow Springs, OH, USA; 6600 V2-4) measured hourly profiles of dissolved oxygen and temperature, and these observations were used to assess progression of lake mixing (data not shown). The buoy was also outfitted with a thermistor string (Apprise Technologies, Inc., Duluth, MN, USA) and a sensor that measured aqueous and atmospheric carbon dioxide concentrations every 2 h (Browne, 2004). The mixing manipulation continued until the surface and bottom-water temperatures differed by less than the accuracy of the thermistors (0.2 °C), on 10 July 2008 (the last day of mixing, referred to throughout as Mix), signifying that complete mixing had been achieved.
Samples were collected weekly or twice weekly during June, July and August of 2007, 2008 and 2009, respectively, for standard water chemistry and microbial community analyses. Manual measurements of oxygen and temperature were collected at every sampling time (Yellow Springs Instrument 550 A) and used to identify the thermocline. Integrated epilimnion and hypolimnion samples were collected as previously described (Shade et al., 2008). In 2008, integrated thermal-layer samples were collected more frequently (usually daily) during and after the whole-lake manipulation. In 2008 during the experimental period, we also collected depth-discrete epilimnion (0 m) and hypolimnion (4 m) water samples using a peristaltic pump that was rinsed between each sample. Depth-discrete samples were collected in this manner even when the lake was completely mixed (and therefore no epilimnion or hypolimnion layers existed). Thus, on the day that complete mixing was achieved (Mix), we considered the 0 m and 4 m water samples to represent epilimnion and hypolimnion water, respectively, even though the temperature throughout the water column was homogeneous. Collected water was preserved for water chemistry (total and dissolved nitrogen, total and dissolved phosphorus, dissolved organic and inorganic carbon, water color) using the North Temperate Lakes Long Term Ecological Research standard protocols (http://lter.limnology.wisc.edu/). Water for bacterial community analysis was filtered onto a 0.2 μm polyethersulfone membrane (Pall, New York, NY, USA), frozen at −20 °C at the field station, and then transferred to −80 °C at the main laboratory in Madison.
During 2008, we also measured concentrations of several terminal electron acceptors and donors that are hallmarks of distinct biogeochemical transformations mediated by microorganisms under anoxic conditions (for example, iron reduction, sulfate reduction and methanogenesis), throughout the ice-free season. Data presented here were collected on 01 July (day −9), 10 July (Mix), 13 July (day 3), 15 July (day 5), 17 July (day 7), 19 July (day 9), 21 July (day 11) and 30 July (day 20). All parameters were measured at 4 m. We also measured five-point water column profiles of carbon dioxide and methane. For the carbon dioxide and methane profiles, one sample was always collected at 0 m, one sample was always collected at 4 m to match the microbial samples, and three additional samples were collected at intermediate depths that best characterized the inflection points in dissolved oxygen over the oxycline. These middle three sample locations varied with the thermal and oxygen structure of the water column, and therefore were not the same depths across all sampling times. When the water column was mixed (no oxygen inflection point was observed), methane and carbon dioxide samples were collected equidistant from the surface and bottom at 0, 1, 2, 3 and 4 m.
Water chemistry
Water chemistry analyses were performed by the North Temperate Lakes Long Term Ecological Research, according to their standard protocols (http://lter.limnology.wisc.edu/), except for iron, sulfide, carbon dioxide and methane. Samples for dissolved Fe were acidified with HCl to pH<2 and analyzed colorimetrically using ferrozine (Stookey, 1970). Samples for dissolved sulfide were stabilized by addition of 1% (wt vol−1) zinc acetate (to convert dissolved sulfide to ZnS), and analyzed colorimetrically via the methylene blue method (Cline, 1969). Carbon dioxide and methane were measured using gas chromatography (Shimadzu GC-14A, Kyoto, Japan), equipped with a flame ionization detector and a methanizer, which allowed for analysis of both CH4 and CO2 (which is converted to CH4 by a methanizer) in a single injection.
Bacterial community sequencing and fingerprinting
Total DNA was extracted from frozen filters as described using the FastPrep kit (Qbiogene, Carlsbad, CA, USA) with minor modifications (Fisher and Triplett, 1999). To track bacterial community responses to mixing, we employed a bar-coded pyrosequencing approach targeting the V2 variable region of the 16S ribosomal RNA gene (Fierer et al., 2008; Hamady et al., 2008) to process samples collected from the epilimnion (0 m) and hypolimnion (4 m) on 01 July (day −9), 10 July (Mix), 13 July (day 3), 15 July (day 5), 17 July (day 7), 19 July (day 9), 21 July (day 11) and 30 July (day 20). Pyrosequencing was performed using previously-described protocols for sequencing (Fierer et al., 2008), quality control, phylogenetic assignment (Hamady et al., 2010) and calculation of phylogenetic diversity (Faith, 1992). The sequencing targeted 250 base-pairs of the 16S ribosomal RNA gene using the primers Pyro 27f and 338R. We recovered an average of 1441 sequences per sample (range 1248–1672). The phylogenetic tree was built using FastTree (Price et al., 2009). Analyses were performed with QIIME (Caporaso et al., 2010), using default parameters including the RDP classifier (Wang et al., 2007) for taxonomy assignment and cd-hit (Li and Godzik, 2006) with a 97% threshold for operational taxonomic unit (OTU) picking. Communities were standardized by total number of reads.
Rarefied Faith’s phlyogenetic diversity (PD, (Faith, 1992)) and rarefied richness were calculated in QIIME. Pielou’s Evenness, J, was calculated based on Shannon diversity, H (J=H/log (richness)). The Grubb’s outlier test (outliers package (Komsta, 2011)) in the R environment for statistical computing (R Development Core Team, 2010) was used to determine if the day of complete mixing values of richness, Pielou’s evenness or PD were significantly different from the rest of the observations in the series. Grubb’s test calculates a score for the outlier by subtracting the proposed outlier value from the set mean and then dividing by the s.d. (G statistic), and also by comparing the ratio of variances of the set with and without the proposed outlier included (U statistic). A paired, two-sided t-test was used to determine if epilimnion and hypolimnion trajectories of richness, Pielou’s evenness or PD were significantly different from each other.
Non-metric multidimensional scaling analysis was performed with UniFrac weighted distances (Lozupone et al., 2007) vegan package in R (Oksanen et al., 2011). The results are not sensitive to choice of metric: in this case, weighted UniFrac performed similarly to unweighted UniFrac and also Bray–Curtis similarity, suggesting that for this ecosystem patterns in community membership and community structure are highly correlated (see Supplemental Online Materials). The R environment was used for calculation of evenness and creation of bar charts and heatmaps by phyla. The vegan package in R was used for correspondence analysis, Mantel tests and Procrustes analysis.
For the evaluation of the species abundance distributions (Magurran, 2007), we fitted log-normal models to the ranked abundance of each taxon in the pyrosequencing data, assuming an underlying Poisson distribution (vegan package in R). Null, pre-emptive, log-normal, Zipf and Zipf-Mandelbrot models were each fit to the data (Wilson, 1991). Null and pre-emptive models did not produce good fits, as evaluated by Akaike information criterion, Bayesian information criterion and deviance. However, the remaining models had comparable fits. The log-normal model was selected because it made the fewest assumptions about the underlying ecology of the data. Species abundance distributions for other models reproduced the same shift in the community structure at the day of mixing.
To examine bacterial community change before, during and after the mixing event, we used automated ribosomal intergenic spacer analysis (ARISA) to fingerprint bacterial communities from integrated epilimnion and integrated hypolimnion water samples (Fisher and Triplett (1999) collected during June, July and August, with modifications provided in Shade et al. (2011)). This resulted in ARISA profiles for the following samples: 17 in 2007, 60 samples in 2008 and 17 in 2009. Note that this integrated sampling strategy was different than what was used for the depth-discrete samples that were pyrosequenced. The demarcation of epilimnion and hypolimnion samples was determined by thermal profiles measured at the time of sampling, and the thermocline was included in the epilimnion sample. Thus, integrated epilimnion and integrated hypolimnion together included the whole-lake column.
ARISA profiles from integrated thermal-layer samples were used to determine to what extent physical displacement of OTUs via water motion could explain observed changes in community composition, during the July 2008 manipulation. We compared day −10 samples (30 June 2008) with Mix (10 July 2008) to assess the proportion of Mix community members that could be attributed to OTUs present in the epilimnion or hypolimnion before the water column was mixed. Any community members that were present in the Mix sample but were not observed in the pre-mixing samples were designated as ‘Unknown origin.’ We used a Venn analysis, pie charts and hierarchical clustering with a heatmap of OTU occurrences to visualize differences in pre-mix and Mix communities (R Development Core Team, 2010).
Next, we performed simulations of community richness and evenness to determine the probability of observing the Mix community given the pre-mix composition. For the simulation, we used re-sampling without replacement of integrated epilimnion and integrated hypolimnion communities in ‘mixing scenarios’ of the following proportions (epilimnion/hypolimnion contribution): 10/90, 20/80, 30/70, 40/60, 50/50, 60/40, 70/30, 80/20 and 90/10 (Supplemental Methods). For 1000 resamplings at each scenario, we report a P-value to describe how often we encountered the observed Mix richness and evenness in the simulated re-sampling. Using the ARISA data from the integrated water samples, we could evaluate whether the community composition at Mix could be explained by different degrees of physical displacement of community members. Richness was compared rounded with the nearest whole number, and evenness was compared with three significant digits. More details for this analysis are provided in Supplemental Materials.
We also used ARISA fingerprints to compare inter-annual dynamics in North Sparkling Bog the year before (2007, June through October) and the year after the manipulation (2009, June and July). These samples were fingerprinted from integrated epilimnion and integrated hypolimnion water. If the lake bacterial communities recovered after the manipulation, we would expect the dynamics after the manipulation to be generally consistent across years because of the seasonality previously observed in North Temperate bog bacterial communities (Kent et al., 2007; Shade et al., 2008). To assess community variability, we calculated permuted analysis of betadispersion of pairwise Bray–Curtis similarities (vegan package (Oksanen et al., 2011) and (Anderson et al., 2006)). This analysis calculates dispersion from a mean compositional centroid within a categorical group. In this case, groups were months within years (for example, July 2007). As epilimnion and hypolimnion communities vary differently through time (Shade et al., 2007), epilimnion and hypolimnion samples were analyzed separately.
ARISA fingerprints were further used to calculate a rate of compositional change (a.k.a. similarity-decay) in the community. The slope of the log-linear regression between Bray–Curtis dissimilarity and days between observations is a rate of compositional change (Nekola and White, 1999; Soininen et al., 2007); this is similar to the strategy used to assess rate of change in the previous mesocosm experiment (Shade et al., 2011). We compared rates across the 2007–2009 June–August time series to the 2008 post-mixing time series (July–August), and also to the rate of change in temperature over the same time period. The temperature rate was assessed in the same way, by regressing the change in temperature against the time between observations.
Results
Physical and chemical changes after mixing
Artificial mixing continued for 8 days until temperature was the same throughout the water column. As compared with the previous ice-free season (2007), mixing disrupted the normal physical and chemical structure of the lake, creating a pulse disturbance and exposing bacterial communities to markedly different environmental conditions. These different conditions included increased bottom-water temperature from 6 to 20°C and increased dissolved oxygen concentration from below detection (<0.01 mg l−1) to near 3 mg l−1 (Figures 1a and b). The vertical oxygen gradient was homogenized gradually, but then returned within 2 days to its pre-manipulation anoxic conditions (12 July 2008, day 2) (Figures 1c).
Temperature and dissolved oxygen profiles of North Sparkling Bog during the year before mixing (2007; a, c) and the year of the mixing manipulation (2008; b, d). Profiles were observed with a hand-held dissolved oxygen and temperature sensor. Mixing began on 02 July 2008 (day −8) and ended on 10 July 2008 (day 0, Mix) when homogenous temperature was observed. Black ticks above panels indicate sampling points.
We consider ‘recovery’ as the point in which the biology or chemistry matched the pre-mixing time point, day −9. During the recovery period of the lake, stratification was relatively weak and more susceptible to weather-induced mixing, which is not typical for this typically strongly stratified lake (Figure 1a). We observed a spontaneous secondary mixing event 4 days after treatment had ceased (day 4). This secondary event occurred because overall the manipulation warmed the lake and created nearly isothermal conditions. The lack of thermal stability in the water column made the lake unusually susceptible to weather events that encourage mixing, such as temperature changes or wind. To the best of our knowledge (and based on inspection of thermal profiles collected from the time that instrumented buoys were first deployed on similar lakes, available at http://www.gleon.org), a spontaneous, full water column mixing event has never been observed in mid-summer in North Sparkling Bog.
To understand any possible functional microbial responses that may be attributed to lake mixing, we measured the concentrations of various hypolimnion respiration products over the mixing experiment. Measurements of hypolimnion iron (Fe2+ and FeTotal), sulfur (H2S) and nitrogen (NH4) respiration products decreased to a minimum on the day complete mixing was achieved (Mix), and then rapidly increased, generally following the overall response of dissolved oxygen (Figure 2a). SO4 had a ‘lag’ response that correlated with the post-mixing increase in H2S. Additionally, Fe3+ and NO3 did not vary substantially over the course of the experiment, and were both present in comparably low concentrations.
Changes in measured water chemistry before and after lake mixing. (a) Microbial oxidation–reduction chemistry measured at 4 m depth (hypolimnion) over the mixing experiment. Pre-mixing, Mix and post-mixing time points are on the x axis. (b) Dissolved gas concentrations over the mixing experiment. Daily average carbon dioxide concentration in the surface waters of North Sparkling Bog during the ice-free period of 2008, measured by a carbon dioxide sensor deployed on the lake. (c) Methane (open symbols) and carbon dioxide (closed symbols) profiles for day −9 (pre-manipulation), for (d) Mix (when complete mixing was achieved), and for (e) day 20 (post-mixing). c–e were measured using standard analyses of depth-discrete, manually-collected samples.
We also considered the dynamics of gases CO2 and CH4, two microbial products, over the course of the mixing experiment. CO2 concentrations in the surface waters during the mixing experiment were markedly higher than CO2 concentrations in the month prior and were more comparable with concentrations observed in the autumn during fall mixing (Figure 2b). Methane and CO2, two products of microbial respiration that often accumulate in the hypolimnia of stratified lakes, were homogenous throughout the water column at low concentrations at Mix, but re-accumulated at the lowest depth (4 m) by day 20 post-mixing (Figures 2c–e).
Comparing patterns in ARISA fingerprinting to patterns in pyrosequencing
We first compared bacterial community composition measurements made with automated ribosomal intergenic spacer analysis (ARISA) to those generated via tag pyrosequencing from the same depth-discrete 0 m and 4 m July 2008 water samples, to ensure that they revealed the same community patterns. Using Bray–Curtis similarity for the ARISA fingerprints and weighted UniFrac distance for the pyrosequencing data, we observed similar patterns and results across both data sets (Mantel Pearson’s R=0.79, P=0.001 on 1000 permutations). We found a similar agreement across data sets by Procrustes correlation between correspondence analyses sample scores (R=0.75, P=0.003 on 1000 permutations). This analysis implied that community patterns generally were consistent across these two methods, and that results from one method are relevant to understanding results from the other (Supplemental Materials).
Changes in bacterial diversity after mixing
To characterize community robustness, we analyzed important ecological aspects of community diversity: richness (number of distinct taxa), evenness (equitability of taxa), structure (representation of dominant and rare taxa), PD, the number and relationship of phylogenetic lineages) and composition (relative abundance of specific taxa defined as phylotypes at the 97% sequence similarity level), using the tag pyrosequence data. Changes in community composition, loss of species richness and gain in species richness relative to the pre-mix conditions (possible bloom or growth of populations) were used as indicators of community responses to the disturbance. July 2008 (the month of the mixing experiment) samples from water collected at depth 0 m (epilimnion) and depth 4 m (hypolimnion) were used to assess immediate community responses to mixing.
We predicted that the epilimnion (0 m) and hypolimnion (4 m) communities would be indistinguishable under mixed conditions. However, richness and evenness were different, whereas PD was comparable across depths (Figure 3). This suggested that despite thermal homogeneity of the lake, each stratum maintained a separate community structure, possibly driven by species sorting by environmental drivers other than temperature. Such a situation could arise if the time scale of vertical water movement (likely driven by convective mixing rather than wind, (Read et al., 2012)) was slower than growth or loss rates.
Bacterial response to lake mixing, assessed with bar-coded pyrosequencing of the 16S rrn gene V1–V2 region and analyzed for changes in epilimnion (0 m, open symbols) and hypolimnion (4 m, closed symbols) community (a) richness, (b) Pielou’s evenness and (c) PD. Error bars on the rarefied values are too small to visualize on the chart; overlap in s.ds. between epilimnion and hypolimnion values occurred on Mix for PD and on day 3 for richness.
For each aspect of diversity evaluated and for each depth (0 and 4 m) of the lake, the bacterial communities recovered within days to pre-perturbation states (Figure 3, Figure 4). There was an opposite response in diversity in each of the two stratum (Figure 3). The epilimnion communities became richer and included taxa that were more phylogenetically divergent after the mixing (Mix). All measures of epilimnion diversity peaked on day 3 post-mixing, and then returned to pre-mixing levels (Figure 3, open symbols). Pielou’s evenness on day 3 post-mixing was detected as significantly different from the remaining July 2008 time series by the Grubb’s outlier test (G=2.04, U=0.002, P<0.001). Together, these data suggest that the maximal change of the epilimnion community to the mixing experiment occurred not on the day of complete mixing, but after a 3-day lag.
Log-normal models fit to ranked taxa abundance to assess changes in the rarity and dominance of members in the (a) epilimnion (0 m) and (b) hypolimnion (4 m). Red arrows below the x axis show the shift in community structure after mixing, and black arrows show recovery. Each line color is a community structure at a different sampling time. In the epilimnion, communities experienced a second, greater shift in structure on day 3, shown by the two red arrows.
In comparison, the hypolimnion communities were less rich (Grubb’s G=1.97, U=0.072, P=0.006), contained fewer phylotypes (G=1.93, U=0.102, P=0.012), and were less even (G=3.145, U=0.017, P<0.001) on Mix, but rapidly recovered to pre-mixing levels by day 3 (Figure 3, closed symbols). These analyses suggest that the hypolimnion community changed maximally around Mix. As an overall comparison of the diversity time series, epilimnion and hypolimnion communities were different in evenness and PD, but they were not different in richness, suggesting that though the number of OTUs observed at either depth were comparable, each depth contained a different representation of taxa (evenness T=2.93 P<0.05; PD T=−3.64, P<0.05; richness P=0.27)
Species abundance distributions were used to investigate changes in the bacterial communities’ structures (Magurran, 2007; McGill et al., 2007). There was a strong shift in the structures of both epilimnion and hypolimnion communities (Figure 4). The epilimnion became more even (taxa shared similar relative abundances) during and immediately after the manipulation and the largest shift was between Mix and day 3 (Figure 4a). The hypolimnion became less even (loss of richness and dominance by a few taxa, Figure 4b) during the manipulation, and largely recovered by day 3. The recovery of the shape of the species abundance distributions further supports community recovery.
Changes in bacterial community composition after mixing
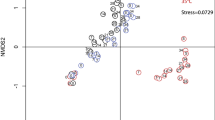
We next assessed changes in epilimnion and hypolimnion composition (defined as the relative abundances of OTUs) before and after lake mixing, using tag pyrosequence data (Figure 5). The epilimnion community experienced a large change between day −9 pre-mixing and Mix, a second smaller change between Mix and day 3 post mixing, and then another large change between days 3 and 7 post-mixing (Figure 5a). After day 7, the post-mixing epilimnion community was most similar in composition to the pre-mixing state, suggesting epilimnion community recovery (weighted UniFrac distance between epilimnion day −9 and day 7=0.13, Supplementary Figure 1a).
Changes in bacterial community composition assessed with pyrosequencing and visualized by non-metric multidimensional scaling analysis (NMDS) of weighted UniFrac similarities in the (a) epilimnion (0 m), (b) hypolimnion (4 m), (c) epilimnion (red lines) and hypolimnion (blue lines). The communities change along trajectories shown by the dashed lines. Orange circles identify the pre-mix time point (day −9), green circles identify the day complete mixing was achieved (Mix), and yellow circles identify the recovery time point (day 20). (d) Differences in weighted UniFrac distance between the epilimnion (0 m) and hypolimnion (4 m) through the experiment. Note that epilimnion and hypolimnion are operationally defined on Mix because strata do not exist when water column temperature is homogeneous.
The hypolimnion community in North Sparkling Bog changed directionally until it returned to a composition similar to pre-mixing by day 11 post-mixing (Figure 5b; weighted UniFrac distance between hypolimnion day −9 and day 11=0.17; Supplementary Figure 1b). As described above, the epilimnion and hypolimnion communities became less distinct during mixing (weighted UniFrac distance=0.15), but then diverged quickly as thermal stratification was re-established (Figures 5c). Epilimnion and hypolimnion communities remained 15% different by weighted UniFrac distance, though the dissimilarity between communities at the two depths reached a minimum at Mix (Figure 5d). Further, similarity between the epilimnion day 20 and day −9 communities was greater than the similarity between the same days in the hypolimnion, suggesting that the epilimnion community recovered more completely than the hypolimnion.
There were distinct responses to mixing among phylogenetically coherent groups defined at the phylum or Proteobacteria class level (Figure 6). In the epilimnion, Gamma-Proteobacteria bloomed briefly on day 3 post-mixing, possibly responding to nitrogen or other nutrients up-welled from the hypolimnion after a lag for growth (Supplementary Figure 2), suggesting that there were community changes that could be attributed to biology. In the hypolimnion, populations of Acidobacteria and Delta-Proteobacteria were below detection at Mix, but re-appeared by day 3. Several members of these latter phyla are strict anaerobes, and may have either died or were protected within a micro-niche when the hypolimnion was oxygenated. Bacteriodetes also decreased in the hypolimnion and gradually recovered within 20 days. Other phyla, such as hypolimnion Chlorobi and epilimnion Firmicutes, had dynamics that did not obviously correspond to the mixing experiment.
Phylum-level (and Proteobacterial class level) changes in epilimnion (0 m) and hypolimnion (4 m) composition through the lake mixing experiment. Relative abundances of phylotyped reads are shown, identified to their closest match in the Ribosomal Database Project. Asterisks indicate that no members associated with the phylum were detected. In the x axis, orange circles identify the pre-mix time point (day −9), green circles identify the day complete mixing was achieved (Mix) and yellow circles identify the recovery time point (day 20). See Supplementary Figure 3 for a different perspective of phylum-level dynamics, normalized by total occurrences within each phylum to visualize maximum changes.
To compare similar patterns of change across phyla, we standardized each phylum’s dynamics to its maximum observed abundance (for example, a dominant phylum that changed twofold would be scaled the same as a rare phylum that also changed twofold, Supplementary Figure 3). In the epilimnion, members of less-abundant phyla generally responded to mixing more dramatically than dominant phyla. In the hypolimnion, some rare phyla (for example, Gamma-Proteobacteria and Firmicutes) bloomed after the mix, while some abundant phyla (for example, Bacteroidetes and Acidobacteria) dropped below detection on Mix and recovered by day 3 post-mixing.
Understanding community changes at mixing: displacement and ecology
To determine the separate contributions of physical displacement of cells and biological processes (that is, active migration or growth of OTUs), we used ARISA fingerprints of integrated epilimnion and integrated hypolimnion water samples (Figure 7). ARISA OTUs that were not detected before the manipulation (day −10, note that this is 1 day earlier that the day −9 time point used for pyrosequencing) were unique to the ‘Epi Mix’ and ‘Hypo Mix’ communities (Figure 7a). These unique Mix OTUs were of unknown origin, and represented 11% of the epilimnion and 12% of the hypolimnion communities (Figure 7b). Further, the epilimnion mix and hypolimnion mix communities were more similar to each other than they were to either the epilimnion or the hypolimnion community before mixing (Figure 7c).
Comparison of composition across integrated epilimnion and integrated hypolimnion communities fingerprinted with ARISA, collected 2 days before the mixing manipulation began (day −10) and on the day that complete water column mixing was achieved (Mix). (a) ARISA OTUs detected in and shared across the epilimnion and hypolimnion on day −10 and Mix. Numbers in parentheses show the proportion of detected OTUs belonging to the group. (b) For the epilimnion and hypolimnion Mix communities, the proportion of OTUs that originated in the epilimnion on day −10, OTUs that originated in the hypolimnion on day −10, OTUs that were associated with both layers on day −10, and OTUs that were uniquely observed on the day of Mix (labeled as ‘unknown origin’). (c) Heatmap and cluster analysis of community composition of epilimnion and hypolimnion day −10 and epilimnion and hypolimnion Mix. OTUs are in columns (ARISA OTU IDs are provided along the x axis) and rows are separate communities. OTUs are clustered based on similar abundance and occurrence patterns across the four samples.
Finally, we performed re-sampling of the day −10 communities in various scenarios of mixing to assess how likely it was to observe the Mix communities given the day −10 composition. We re-sampled communities one thousand times, including epilimnion and hypolimnion proportions of 10/90, 20/80, 30/70, 40/60 and 50/50, and assessed richness (assessed by the number of unique ARISA fragment lengths) and Pielou’s evenness for each re-sampling. For these tests (a total of nine mixing scenarios of different proportions of epilimnion and hypolimnion contribution), we never observed the actual ARISA-based richness and evenness after 1000 resamplings at each mixing scenario (Supplementary Figure 4). Together, these results suggest that there were changes in composition that could not be attributed only to physical displacement of cells, but that may be instead explained by ecological processes.
Long- and short-term effects on bacterial community dynamics
To evaluate whether the mixing experiment caused prolonged changes in bacterial community dynamics, we assessed seasonal variability using community ARISA fingerprints from 2007 through July 2009. Because bacterial communities in north temperate bog lakes were previously shown to change seasonally (Kent et al., 2007; Shade et al., 2008) we used permuted analysis of betadispersion of Bray–Curtis similarities to address whether there were overall monthly differences in variability between the year of mixing and the other 2 years. We detected no differences in overall variability across months and years (epilimnion P=0.35 on 999 permutations, hypolimnion P=0.10, Supplementary Figure 5). This suggests that the mixing experiment in July 2008 did not have prolonged or permanent effects on bacterial community dynamics.
We then asked whether the mixing manipulation influenced the bacterial community rate of change, by comparing rates observed in the epilimnia and hypolimnia from 2007, 2008 and 2009. We calculated the rate of change (similarity-decay) by regressing the Bray–Curtis dissimilarity (calculated using ARISA profiles) against the time interval between samples, for all possible pairs of samples within each time series, and recording the slope of the regression (Supplementary Figure 6). The hypolimnion communities sampled in 2008 post-mixing changed faster than any of the other communities. The bacterial community rate of change was correlated with the rate of change in temperature (r=0.94, P=0.005, n=6). Taken together, these findings suggest that the elevated temperature in the hypolimnion contributed to a faster rate of community change than is normally observed in the cold bottom waters of this bog lake.
Discussion
We employed a whole-ecosystem manipulation to ask whether microbial communities could recover after a large disturbance, and to ask how responses of microbial communities compared with changes in environmental conditions. Bacterial communities were not resistant to summer mixing, but were highly resilient. The communities in the epilimnion and hypolimnion both changed as a result of the disturbance, but recovered by days 7 and 11 post-disturbance, respectively. Though microbial communities in the epilimnion and hypolimnion had contrasting responses to the disturbance (for example, epilimnion increased in diversity and the hypolimnion decreased), they had similar patterns of recovery. Although the manipulation seemed to increase the rate of community change in the hypolimnion during summer 2008, the communities did not exhibit prolonged changes in dynamics after the mixing experiment, as assessed by comparing overall variability in the year of mixing to ARISA fingerprints from 2007 through July 2009.
The richness and evenness of the integrated epilimnion and integrated hypolimnion at Mix were not the same as any simulated mixing scenarios, suggesting that vertical transport of cells in the water column was not enough to explain differences between the two layers (between day −10 and Mix). We also observed changes in individual phylotype and OTU relative abundances that could not be explained by cell transport in the water column. For example, at mixing, Alpha-Proteobacteria tags increased in relative abundance in the hypolimnion, while other tags previously abundant in the hypolimnion fell below detection (for example, Acidobacteria, Delta-Proteobacteria). Several members of these latter phyla are strict anaerobes, and were either protected in micro-niches or possibly died when the hypolimnion was oxygenated. Interestingly, the Beta-Proteobacteria decreased in the epilimnion as a proportion of total tags, but changed minimally in the hypolimnion. Thus, we infer that biological processes (for example, growth, death, revival from a dormant state) contributed to the observed temporal dynamics. For example, dormant microorganisms persisting in a sediment seed bank may have a role in community resilience after disturbance (Jones and Lennon, 2010), but further study is needed to determine the role of inactive members, as well as extremely rare members, to overall community recovery.
Hypolimnion response and recovery
Hypolimnion communities changed more drastically than epilimnion in composition, richness and phylogenetic diversity. The recovery of hypolimnion composition coincided with the depletion of dissolved oxygen and the re-accumulation of anaerobic respiration products such as methane, carbon dioxide and other reduced molecules (for example, ferrous iron, ammonium and sulfide). As would be expected, accumulation of sulfide coincided with a decrease in sulfate, several days after Mix. Taken together, these observations are consistent with the transitions from aerobic to anaerobic respiration that we would predict from first-principles of biogeochemistry (for example, electron-donor and -acceptor pairs). Thus, the microorganisms and their respiration products helped to create the environmental conditions that eventually harbored a recovered hypolimnion community. This demonstrates a fundamental feedback loop for the hypolimnion community: microorganisms help to create the strong oxidation–reduction gradients, and this gradient then dictates the observed community robustness to mixing by acting as an environmental filter that drives species sorting.
In our previous mesocosm experiment, the hypolimnion community also recovered as oxygen concentration decreased, despite that temperature immediately recovered to pre-disturbance conditions (Shade et al., 2011). Notably, the hypolimnion temperature was approximately 6 °C during the mesocosm experiment (the same as the ambient hypolimnion temperature) and the community recovered after 10 days, while in the whole-lake manipulation the temperature was 20 °C (elevated as compared with the pre-disturbance 6 °C) and the community recovered after 11 days. This indicates that oxygen concentration is more important than temperature in determining hypolimnion community composition. This does not exclude the possibility that other, unmeasured environmental changes also were important in driving the microbial community, but oxygen proved to be the most important among those that we measured. However, elevated temperatures appeared to accelerate the hypolimnion community changes after mixing. Recent estimates of bacteriovore grazing rates in a similar small bog lake suggested bacterial growth rates were on the order of 0.33 day even at 9 oC (Hahn et al., 2012). This is consistent with the community composition in the recovering hypolimnion turning over during a period of days.
Summer lake mixing as a novel, large disturbance
We have provided several lines of evidence that our mixing experiment represented both a novel and extreme disturbance to lake bacteria. The first is the drastic changes in temperature and oxygen, key drivers of epilimnion and hypolimnion aquatic bacterial communities, respectively (Shade et al., 2011). The temperature in the hypolimnion increased from 6 to 20 °C. The historical ice-free hypolimnion temperature has likely never exceeded 10 °C. The oxygen in the hypolimnion was increased from 0 to near 3 mg/l. Stable bog lakes such as North Sparkling Bog remain anaerobic in the bottom waters with the exception of seasonal mixing (fall and spring). Both of these environmental changes were major deviations from seasonal norms. Second, the known seasonality of the microbial communities in North Sparkling Bog (Kent et al., 2007) provides additional evidence for the novelty of the mixing event. Because North Sparkling Bog bacterial communities typically follow a temperate lake seasonal trajectory, we have observed that community composition in July is very different from the composition observed during spring and fall mixing. Therefore, the mixing disturbance was novel to summer ‘July’ communities. Finally, the duration of the mixing event presents an extreme disturbance for this ecosystem. After 8 days, complete mixing was achieved in the lake, whereas seasonal spring and autumn mixing occur after weeks to months of gradual cooling of the epilimnion and periodic entrainment of these cooled waters into the hypolimnion, until temperature homogeneity is reached through to the sediment of the lake. The experimental mixing occurred over a much shorter period than seasonal mixing.
Conclusions
Microorganisms as sentinels of change
Our results have implications for understanding microbiological responses to environmental perturbations at both fine and coarse temporal scales. In this study, the microbial communities changed after the disturbance, exhibiting sensitivity to a pulse perturbation that occurred over a relatively short time scale (1 week). Further, the clear connection between biogeochemical conditions and community composition suggests that there are direct linkages between changes in environmental forcing, biogeochemical conditions, and microbial communities. The communities recovered within days, and the analysis of variability from 2007 to 2009 demonstrated that the monthly baseline in temporal community variation was relatively stable and may represent a deterministic point of return. Work by our group (Kent et al., 2007; Shade et al., 2007; Shade et al., 2008) and others (Crump and Hobbie, 2005; Fuhrman et al., 2006; Nelson, 2008; Andersson et al., 2010) has shown repeatable seasonal patterns in microbial communities inhabiting rivers, oceans, seas and lakes, providing cumulative evidence of a consistent microbial community ‘baseline’ that spans many aquatic systems. In ecology, increases in ecosystem variance have been suggested as indicators of ecological regime shifts (for example, Carpenter and Brock, 2006). Therefore, gradual changes in aquatic microbial communities that exceed normal variability may correspond to press disturbances, such as those expected with gradual global change (for example, increases in mean temperature). Meanwhile, more dramatic microbial shifts may signal pulse disturbances, such as extremes in precipitation or temperature associated with expected increases in climate variability (Folland et al., 2002). The current work and these previous studies together indicate that consistent time series of aquatic microbial communities may serve as robust sentinels over multiple time scales, from local pulse disturbances to global presses.
References
Allison SD, Martiny JBH . (2008). Resistance, resilience, and redundancy in microbial communities. PNAS 105: 11512–11519.
Anderson MJ, Ellingsen KE, McArdle BH . (2006). Multivariate dispersion as a measure of beta diversity. Ecol Lett 9: 683–693.
Andersson AF, Riemann L, Bertilsson S . (2010). Pyrosequencing reveals contrasting seasonal dynamics of taxa within Baltic Sea bacterioplankton communities. ISME J 4: 171–181.
Arhonditsis GB, Brett MT . (2004). Evaluation of the current state of mechanistic aquatic biogeochemical modeling. Mar Ecol Prog Ser 271: 13–26.
Browne BA . (2004). Pumping-induced ebullition: A unified and simplified method for measuring multiple dissolved gases. Environ Sci Technol 38: 5729–5736.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336.
Carpenter S, Brock W . (2006). Rising variance: a leading indicator of ecological transition. Ecol Lett 9: 311–318.
Cline JD . (1969). Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14: 454–458.
Crump BC, Hobbie JE . (2005). Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50: 1718–1729.
Cytryn E, Minz D, Oremland RS, Cohen Y . (2000). Distribution and diversity of archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl Environ Microb 66: 3269–3276.
Faith DP . (1992). Conservation evaluation and phylogenetic diversity. Biol Conserv 61: 1–10.
Fenchel T, Finlay B . (2008). Oxygen and the Spatial Structure of Microbial Communities. Biol Rev 83: 553–569.
Fierer N, Hamady M, Lauber C, Knight R . (2008). The influence of sex, handedness, and washing on the diversity of hand surface bacteria. PNAS 105: 17994.
Fisher MM, Triplett EW . (1999). Automated approach for ribosomal intergenic spacer analysis of microbial diversity and its application to freshwater bacterial communities. Appl Environ Microb 65: 4630–4636.
Folland CK, Karl TR, Jim Salinger M . (2002). Observed climate variability and change. Weather 57: 269–278.
Fraterrigo J, Rusak J . (2008). Disturbance-driven changes in the variability of ecological patterns and processes. Ecol Lett 11: 756–770.
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S . (2006). Annually reoccurring bacterial communities are predictable from ocean conditions. PNAS 103: 13104.
Glasby T, Underwood A . (1996). Sampling to differentiate between pulse and press perturbations. Environ Monit Assess 42: 241–252.
Hahn MW, Scheuerl T, Jezberova J, Koll U, Jezbera J, Simek K et al (2012). The passive yet successful way of planktonic life: genomic and experimental analysis of the ecology of a free-living polynucleobacter population. PLoS One 7: e32772.
Hamady M, Lozupone C, Knight R . (2010). Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4: 17–27.
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R . (2008). Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nature Methods 5: 235–237.
Heaney SI, Talling JF . (1980). Dynamic aspects of dinoflagellate distribution patterns in a small productive lake. J Ecol 68: 75–94.
Jones SE, Chiu CY, Kratz TK, Wu JT, Shade A, McMahon KD . (2008). Typhoons initiate predictable change in aquatic bacterial communities. Limnol Oceanogr 53: 1319–1326.
Jones SE, Lennon JT . (2010). Dormancy contributes to the maintenance of microbial diversity. PNAS 107: 5881–5886.
Katz RW, Brown BG . (1992). Extreme events in a changing climate: variability is more important than averages. Climatic change 21: 289–302.
Kent AD, Yannarell AC, Rusak JA, Triplett EW, McMahon KD . (2007). Synchrony in aquatic microbial community dynamics. ISME J 1: 38–47.
Komsta L . (2011). outliers: Tests for outliers. v. R package version 0.14.
Li WZ, Godzik A . (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659.
Lozupone CA, Hamady M, Kelley ST, Knight R . (2007). Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microb 73: 1576–1585.
Magurran AE . (2007). Species abundance distributions over time. Ecol Lett 10: 347–354.
McGill BJ, Etienne RS, Gray JS, Alonso D, Anderson MJ, Benecha HK et al (2007). Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol Lett 10: 995–1015.
McGuire KL, Treseder KK . (2010). Microbial communities and their relevance for ecosystem models: Decomposition as a case study. Soil Biol Biochem 42: 529–535.
Nekola JC, White PS . (1999). The distance decay of similarity in biogeography and ecology. J Biogeogr 26: 867–878.
Nelson CE . (2008). Phenology of high-elevation pelagic bacteria: the roles of meteorologic variability, catchment inputs and thermal stratification in structuring communities. ISME J 3: 13–30.
Oksanen JF, Blanchet G, Kindt R, Legendre P, Minchin PR, O'Hara R et al (2011). vegan: Community Ecology Package. v. R package version 2. 0–2.
Ovreas L, Forney L, Daae FL, Torsvik V . (1997). Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microb 63: 3367–3373.
Pimm S . (1984). The complexity and stability of ecosystems. Nature 307: 321–325.
Price MN, Dehal PS, Arkin AP . (2009). FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26: 1641–1650.
R Development Core Team (2010) R: A language and environment for statistical computing. v. 2.12.
Read J, Shade A, Wu C, Gorzalski A, McMahon KD . (2011). ‘Gradual Entrainment Lake Inverter’ (GELI): A novel device for experimental lake mixing. Limnol Oceanography: Methods 9: 14–28.
Read JS, Hamilton DP, Desai AR, Rose KC, MacIntyre S, Lenters JD et al (2012). Lake-size dependency of wind shear and convection as controls on gas exchange. Geophysical Res Lett 39: L09405.
Romme W, Everham E, Frelich L, Moritz M, Sparks R . (1998). Are large, infrequent disturbances qualitatively different from small, frequent disturbances? Ecosystems 1: 524–534.
Sarmento H, Montoya JM, Vazquez-Dominguez E, Vaque D, Gasol JM . (2010). Warming effects on marine microbial food web processes: how far can we go when it comes to predictions? Philos Trans R Soc B-Biol Sci 365: 2137–2149.
Schindler DW . (1998). Replication versus realism: The need for ecosystem-scale experiments. Ecosystems 1: 323–334.
Shade A, Chiu CY, McMahon K . (2010a). Differential bacterial dynamics promote emergent community robustness to lake mixing: an epilimnion to hypolimnion transplant experiment. Environ Microbiol 12: 455–466.
Shade A, Chiu CY, McMahon KD . (2010b). Seasonal and episodic lake mixing stimulate differential planktonic bacterial dynamics. Microbial Ecol 59: 546–554.
Shade A, Jones SE, McMahon KD . (2008). The influence of habitat heterogeneity on freshwater bacterial community composition and dynamics. Environ Microbiol 10: 1057–1067.
Shade A, Kent AD, Jones SE, Newton RJ, Triplett EW, McMahon KD . (2007). Interannual dynamics and phenology of bacterial communities in a eutrophic lake. Limnol Oceanogr 52: 487–494.
Shade A, Read JS, Welkie DG, Kratz TK, Wu CH, McMahon KD . (2011). Resistance, resilience and recovery: aquatic bacterial dynamics after water column disturbance. Environ Microbiol 13: 2752–2767.
Soininen J, McDonald R, Hillebrand H . (2007). The distance decay of similarity in ecological communities. Ecography 30: 3–12.
Sousa WP . (1984). The role of disturbance in natural communities. Annu Rev Ecol Syst 15: 353–391.
Stookey LL . (1970). Ferrozine—a new spectrophotometric reagent for iron. Analytical Chemistry 42: 77–781.
Turner M, Baker W, Peterson C, Peet R . (1998). Factors influencing succession: lessons from large, infrequent natural disturbances. Ecosystems 1: 511–523.
Turner M, Dale V . (1998). Comparing large, infrequent disturbances: what have we learned? Ecosystems 1: 493–496.
Vincent WF, Neale PJ, Richerson PJ . (1984). Photoinhibition—algal responses to bright light during diel stratification and mixing in a tropical alpine lake. J Phycol 20: 201–211.
Wang Q, Garrity GM, Tiedje JM, Cole JR . (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb 73: 5261–5267.
White PS, Jentsch A . (2001). The search for generality in studies of disturbance and ecosystem dynamics. In: Esser K, Luttge U, Kadereit JW, Beyschlag W (eds). Progress in Botany, vol. 62. Springer: New York, pp 399–450.
White PS, Pickett STA . (1985) The Ecology of Natural Disturbance and Patch Dynamics. Academic Press: Orlando, FL.
Wilson JB . (1991). Methods for fitting dominance/diversity curves. J Veg Sci 2: 35–46.
Acknowledgements
We thank the North Temperate Lakes Microbial Observatory summer 2007, 2008 and 2009 field crews, UW-Trout Lake Station, the UW Center for Limnology and the Global Lakes Ecological Observatory Network for field and logistical support. We thank Luke Winslow for buoy support, especially with the carbon dioxide sensor. We thank YS Dufour, AR Ives, J Handelsman, H Goodrich-Blair, MA Moran and P Weimer for comments on earlier versions of this work. This work was supported in part by the National Science Foundation via grants DBI-0446017 to TKK; MCB-0702395 (Microbial Observatories) to KDM; DEB-0910297 (Doctoral Dissertation Improvement Grant) to KDM and AS; EAR-0525510 (Biogeosciences) to EER; and the North Temperate Lakes Long Term Ecological Research (NTL-LTER) Site (DEB-0822700); grant WR07R007 from the Wisconsin Groundwater Coordinating Council to EER; the Gordon and Betty Moore Foundation to TKK; and the Howard Hughes Medical Institute to RK. AS is a Gordon and Betty Moore Foundation Fellow of the Life Sciences Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shade, A., Read, J., Youngblut, N. et al. Lake microbial communities are resilient after a whole-ecosystem disturbance. ISME J 6, 2153–2167 (2012). https://doi.org/10.1038/ismej.2012.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2012.56
Keywords
This article is cited by
-
Functionally diverse microbial communities show resilience in response to a record-breaking rain event
ISME Communications (2022)
-
Successional dynamics and alternative stable states in a saline activated sludge microbial community over 9 years
Microbiome (2021)
-
Recovery of freshwater microbial communities after extreme rain events is mediated by cyclic succession
Nature Microbiology (2021)
-
Thermal discharge-induced seawater warming alters richness, community composition and interactions of bacterioplankton assemblages in a coastal ecosystem
Scientific Reports (2021)
-
Metabolic cooperation and spatiotemporal niche partitioning in a kefir microbial community
Nature Microbiology (2021)