Abstract
Synechococcus sp. represents an ecologically diverse group of cyanobacteria found in numerous environments, including hot-spring microbial mats, where they are spatially distributed along thermal, light and oxygen gradients. These thermophiles engage in photosynthesis and aerobic respiration during the day, but switch to fermentative metabolism and nitrogen fixation at night. The genome of Synechococcus OS-B′, isolated from Octopus Spring (Yellowstone National Park) contains a phn gene cluster encoding a phosphonate (Phn) transporter and a C–P lyase. A closely related isolate, Synechococcus OS-A, lacks this cluster, but contains genes encoding putative phosphonatases (Phnases) that appear to be active only in the presence of the Phn substrate. Both isolates grow well on several different Phns as a sole phosphorus (P) source. Interestingly, Synechococcus OS-B′ can use the organic carbon backbones of Phns for heterotrophic growth in the dark, whereas in the light this strain releases organic carbon from Phn as ethane or methane (depending on the specific Phn available); Synechococcus OS-A has neither of these capabilities. These differences in metabolic strategies for assimilating the P and C of Phn by two closely related Synechococcus spp. are suggestive of niche-specific constraints in the evolution of nutrient assimilation pathways and syntrophic relationships among the microbial populations of the hot-spring mats. Thus, it is critical to evaluate levels of various P sources, including Phn, in thermally active habitats and the potential importance of these compounds in the biogeochemical cycling of P and C (some Phn compounds also contain N) in diverse terrestrial environments.
Similar content being viewed by others
Introduction
Phosphorus (P) is essential for cell growth, as it is a vital macromolecular constituent of phospholipids, proteins, polysaccharides and cofactors (Torriani-Gorini et al., 1994). Because phosphate minerals are sparingly soluble ([PO4 3] = 1 μM at pH 7, 25 °C), and the geochemical cycling of phosphate is slow, the concentration of orthophosphate is typically the major limiting nutrient in both marine and terrestrial environments (Wu et al., 2000; Mills et al., 2004; Elser et al., 2007). Thus the ability to use alternate P sources is an advantage. For instance, phosphonates (Phns) appear to constitute a significant fraction of dissolved organic P in the oceans. In parts of the Pacific Ocean, Phns can constitute as much as 25% of the dissolved, high-molecular-weight organic P (Clark et al., 1998; Kolowith et al., 2001) although the exact source of the Phns is not known. Phns can be components of membrane phosphonolipids, nucleic acids, proteins and polysaccharides (Kononova and Nesmeyanova, 2002).
Phn use by microbes may be an important and potentially underestimated component of P biogeochemical cycling (Benitez-Nelson et al., 2004; Falkowski et al., 2008; Martinez et al., 2009; Van Mooy et al., 2009). Cyanobacteria in marine habitats (Dyhrman et al., 2006, 2009; Sanudo-Wilhelmy, 2006; Karl et al., 2008; Ilikchyan et al., 2009; Orchard et al., 2009) such as Trichodesmium sp. encode C–P lyases that allow them to use Phns as a P source and as much as 10% of the total internal P can exist as Phns (Dyhrman et al., 2006, 2009). Phns are widely found in terrestrial environments and anthropogenic sources of Phns are ubiquitous. Various Phns are widely used as pesticides, detergent additives, antibiotics and flame retardants (Kononova and Nesmeyanova, 2002; Nowack, 2003; Singh, 2009).
We have obtained complete genome sequences for two related Synechococcus isolates from microbial mats in Octopus Spring, which is located in the Lower Geyser Basin of Yellowstone National Park: Synechococcus OS-A (Syn OS-A) and OS-B′ (Syn OS-B′) (Bhaya et al., 2007). These isolates are differentially distributed along the horizontal thermal gradient of the mat. Syn OS-B′ is prevalent at 53–60 °C whereas Syn OS-A dominates at higher temperatures (58–65 °C) (Allewalt et al., 2006; Kilian et al., 2007). In recent studies we showed that both Syn OS-A and Syn OS-B′ contain an extensive suite of genes in the Pho regulon, which is required for efficient acquisition of P (Supplementary Table S1). This includes a putative operon with 14 genes encoding proteins for phosphonate (Phn) transport (encoded by phnCDE) and C–P lyase activity (encoded by phnG-M), which is present in the Syn OS-B′ genome but lacking in Syn OS-A (Adams et al., 2008). In this study we have examined the ability of the two closely related cyanobacterial isolates, Syn OS-A and Syn OS-B′, to grow on and use different Phn sources, which suggests that there are alternative strategies for metabolizing Phn in the microbial mats. In a complementary approach, we also monitored changes in expression of the cyanobacterial C–P lyase and phosphonatase (Phnase) genes in situ over a diel cycle.
Materials and methods
Culture conditions
Axenic cultures of Syn OS-A (CIW 14) and Syn OS-B′ (CIW 10) were grown as previously described (Adams et al., 2008). Axenic cultures of Syn OS-A (CIW 14) were isolated from original enrichment cultures using the same strategy as described by Kilian et al. (2007). Cultures were grown and maintained at 50 °C in liquid D medium supplemented with 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 8.2–8.3)) and Va vitamins. This growth medium, termed DH10, contains Pi as sodium phosphate at a concentration of 0.77 mM. Pi-free medium (DH10–P) was prepared by replacing the sodium phosphate with an equimolar amount of sodium chloride. Cultures were bubbled with a 3% CO2 in air mixture in a 50 °C incubator, under continuous light (∼75 μmol photon m−2 s−1).
Cells were grown in DH10–P medium supplemented with 0.5 mM methylphosphonate (MePhn), ethylphosphonate (EtPhn) or aminoethylphosphonate (AePhn) obtained from Sigma-Aldrich (St. Louis, MO, USA; MePhn 15730HG, AePhn, 268674–1G and EtPhn, 289876-1G, grade purum). Experiments with Syn OS-B′ were performed with cells acclimated to growth on Phn, as previously described (Adams et al., 2008). Syn OS-A cells were not subjected to an acclimation period.
In situ sample collection, extraction and qRT-PCR
Samples were collected on 28–29 July 2007, from the effluent channels of Octopus Spring at Yellowstone National Park (latitude 44.5340836, longitude −110.7978895) (Steunou et al., 2008). Frozen mat core samples or cells were processed for RNA or DNA extraction as described previously (Steunou et al., 2006, 2008). Briefly, samples for DNA extraction were collected using cork borers to remove an entire core/plug from the mat. Cores were separated into the top green layer (typically between 1 and 3 mm) and the bottom orange layer (typically 2 and 4 mm). The top green layer contains the majority of the cyanobacteria and was used for nucleic acid extraction. Immediately after collection, samples were frozen in liquid nitrogen to prevent nucleic acid degradation.
Genes within the phn operon were amplified from mat samples using specific primers (confirmed by sequencing). Reverse transcription of RNA samples was performed as described previously (Adams et al., 2008). For all experiments a ‘No reverse transcriptase added’ control was carried out. Specific primers were used to amplify segments (∼200 bp) from all cDNAs measured (Supplementary Table S2).
For gene expression analyses (Figure 4), we took cells from cultures bubbled with a 3% CO2 in air mixture in a 55 °C incubator, under continuous light (∼75 μmol photon m−2 s−1). Cells were collected during exponential phase (day 5) after starting a culture at an OD 750 of 0.05. Three biological replicates, each with three technical replicates, were performed to calculate standard error. The level of expression of each gene, when the cells were grown under +P conditions, was considered the control. The rest of the expression values were normalized based on this control, which was taken as 1. For the in situ experiment (Figure 5), we had access to only one biological replicate for each time point (see above for sample processing), but three technical replicates were performed. We determined the absolute value of the RNA encoding a specific gene, among all environmental samples over the diel cycle using primers specific for CYA_1475, CYA_2058 and CYB_0159 (phnC) (Supplementary Table S3). We normalized these values based on the smallest value over the diel cycle for each gene (arbitrarily set at 1). Data are shown as relative units (RU) (Whelan et al., 2003; Steunou et al., 2006). Primers specific for Syn OS-A or Syn OS-B′ genes (Supplementary Table S2) were designed using MetaPrime, a Primer3-based oligonucleotide generation program (Rozen and Skaletsky, 2000); specificity at the 3′ end of the primers allows for the generation of variant-specific oligonucleotides.
Methane and ethane measurements
Cultures were maintained in a sealed vessel in either the light (75 μmol photon m−2 s−1) or dark for various times and the headspace was sampled. Methane and ethane were resolved on a Porapak N column (3 mm internal diameter × 2 m length) with helium as the carrier gas, and quantified using a Shimadzu (Tokyo, Japan) gas chromatograph (GC-8A) equipped with a flame ionization detector. Temperature settings were 60 °C for the column and 100 °C for both the injector and the detector. Calibrations were performed with methane- and ethane-in-nitrogen standards (Scotty II Analyzed Gases, 100 p.p.m.).
Bioinformatics
Putative Phnases were identified in Syn OS-A and Syn OS-B′ by BLAST analyses using previously identified Phnase proteins. Full sequences for known Phnase proteins were obtained from GenBank, and from these, selected sequences were aligned using ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/index.html), hand-edited using the GCG Wisconsin package sequence editor and the Gene.Doc program (http://www.nrbsc.org/gfx/genedoc/).
Results
Growth with Phn as a sole P source
We recently showed that Syn OS-B′ can grow using methyl phosphonate (MePhn) as a sole source of P, but growth was contingent on an acclimation period of approximately 3 weeks in the presence of MePhn (Adams et al., 2008). These acclimated cells attained growth rates on MePhn (as the sole source of P) comparable to growth on phosphate (Pi). Consequently, for all growth experiments described here, we used Syn OS-B′ cells that had been acclimated to MePhn for at least 3 weeks (Adams et al., 2008). This acclimation requirement has been described for several other microorganisms, but it is not understood at the mechanistic level except in Escherichia coli (Iqbal et al., 2004). Syn OS-A cells were not initially subjected to an acclimation regime and it appears that they do not require an acclimation period to be able to use Phns.
We tested the ability of Syn OS-B′ and Syn OS-A to grow on various Phn compounds, including MePhn, EtPhn, phenylphosphonate (PhenylPhn) and AePhn, and the herbicide glyphosate (Hildebrand and Henderson, 1983). To establish maximal growth rates, we grew cells on medium containing Pi, which is generally the preferred P source for microbes. To establish rates of growth when the cells were deprived of P (‘minimum’ growth rates), we used acclimated Syn OS-B′ cells or nonacclimated Syn OS-A cells that were starved for P (that is, grown for 1 week, as they undergo 3–4 doublings, on medium lacking all P before the start of the experiment) (Figure 1). This prestarvation was necessary because these cyanobacteria accumulate high levels of polyphosphate (poly P) (∼39±0.1 nmol poly P per mg of protein) when grown in P-containing medium.
Time course of growth on Phn sources. Growth of acclimated Syn OS-B′ (a) or Syn OS-A (b) at 50 °C in complete DH10 medium (Pi), DH10–P (P starved), or DH10–P supplemented with 0.5 mM MePhn, EtPhn or AePhn. Controls show growth of Syn OS-B′ or Syn OS-A cells maintained in medium lacking P (3 weeks for Syn OS- B′ and 1 week for Syn OS-A) and then, kept in the same medium over the course of the experiment (minimum growth control). Cells per ml are shown on the y axis on a log scale. The P source for each growth curves is given in the figure. The color reproduction of this figure is available on the html full text version of the manuscript.
Both MePhn and EtPhn served as a sole source of P for Syn OS-B′, with rates of growth that were comparable to those observed when Pi was used as the sole P source (Figure 1a). Syn OS-B′ grew slightly slower with fewer doublings (four doublings instead of five) when AePhn was the sole P source. Neither Syn OS-B′ nor Syn OS-A could grow when glyphosate was substituted for Pi in the medium, although certain cyanobacteria can use glyphosate as a sole source of both P and N (Forlani et al., 2008). Unexpectedly, Syn OS-A cells were also able to maintain high rates of growth when various Phn compounds were provided as a sole source of P (Figure 1b), even though they lack the operon encoding the C–P lyase and Phn transporter. In contrast to Syn OS-B′, no acclimation period was required for Syn OS-A. Growth occurred immediately after Syn OS-A was transferred to medium devoid of Pi but supplemented with Phns. Once the internal Pi reserves were depleted, the cells appeared to be able to access the Phn compounds in the medium as a source of P.
Methane and ethane production
The results described above and comparative genomic analysis raised the possibility that Syn OS-A and Syn OS-B′ have different mechanisms for the acquisition of P from Phn. Furthermore, MePhn and EtPhn have the potential to serve as sources of both P and C (Cook et al., 1978; Ternan et al., 1998), and cells that assimilate the P but not the C moiety of Phns might release methane and ethane into the environment (Yakovleva et al., 1998). However, cells that can assimilate the fixed C of the Phn might be able to grow in the dark using Phn as a sole source of reduced carbon. To test these possibilities, we grew Syn OS-B′ on either EtPhn or MePhn in continuous light (75 μmol photon m−2 s−1) in sealed test tubes, and monitored methane or ethane over a period of 168 h by gas chromatography. Methane and ethane accumulated in the headspace when the sole P source was MePhn and EtPhn, respectively (Figures 2a and b). The gas accumulation tracked cell growth as measured by absorbance at 750 nm. Growth of Syn OS-B′ with AePhn as a sole P source in the light also resulted in methane release (no ethane was released), but the final methane concentration achieved in the headspace after 48 h was ∼5-fold lower than for MePhn, and the final cell density was ∼2-fold less (Figure 2c). As expected, neither methane nor ethane was released by cells growing in medium containing Pi but lacking Phn; medium lacking cells also did not release ethane or methane. These results show a direct correlation between cell growth, which requires the acquisition of P from Phn, and the release of ethane, when EtPhn was the sole P source (or methane, when MePhn was the sole P source). Therefore, during photoautotrophic growth of Syn OS-B′, a significant level of reduced C of the Phn was volatilized and not used for cell growth. Syn OS-A, which has neither C–P lyase nor the phn transporter genes, continued to grow in the light with MePhn as its sole source of P, but unlike Syn OS-B′, failed to accumulate methane in the headspace (Figure 2d). Similar results were observed when EtPhn or AePhn was used as the sole P source; growth was sustained but neither methane nor ethane accumulated in the headspace.
Aerobic growth and accumulation of methane or ethane in Phn-supplemented cultures. Squares and solid lines show accumulation of methane (a, c) or ethane (b) and growth of Syn OS-B′ in DH10 (dotted lines) and DH10–P medium supplemented with MePhn (a) or EtPhn (b) or AePhn (c) (solid lines). (d) Methane accumulation (squares) and growth (triangles) of Syn OS-A in DH10 (Pi, circles and dotted lines) and DH10–P medium supplemented with MePhn (MePhn, solid lines). Cells per ml are shown on a log scale at extreme left. The color reproduction of this figure is available on the html full text version of the manuscript.
Syn OS-B′ grown in medium containing both Pi and MePhn (Supplementary Figure S1) preferentially used Pi. For cultures supplemented only with 10 μM MePhn, methane evolution began after a 48–72 h lag, which was extended to >96 h when cultures were supplemented with both 10 μM Pi and 10 μM MePhn. This is likely because the Pi has to be exhausted before the cells initiate significant acquisition and use of Phns. The rates of growth of all cultures on the different sources of P were approximately the same.
Dark growth
Because Syn OS-A and Syn OS-B′ have the potential to use different enzyme complexes for Phn assimilation, it was plausible that there would also be differences in the ways in which these organisms use the C backbones of the Phns (Yakovleva et al., 1998). Syn OS-B′ can grow in the presence of either EtPhn or AePhn in the light as well as in the dark. In the dark (Figure 3), with EtPhn serving as a sole source of fixed C and P, the cells doubled nearly as rapidly and attained approximately the same cell density as light-grown cells over a period of 172 h (∼6–7 doublings). In contrast, acclimated Syn OS-B′ cells did not double in the dark on complete medium (P replete, but with no Phn or organic). We conclude that Syn OS-B′ can use the C backbone of EtPhn and AePhn as a source of fixed C that fuels cell growth in the dark. As shown in Figure 3b, Syn OS-A grew well in the light with EtPhn or AePhn as a sole P source, but neither of these compounds supported the growth of Syn OS-A in the dark (Figure 3). Thus, in contrast to Syn OS-B′ cells, Syn OS-A cells are unable to use Phns as a source of fixed C.
Growth in the light or dark in Phn-supplemented medium. Syn OS-A and acclimated Syn OS-B′ cells grown in continuous light (75 μmol photon m−2 s−1) (gray and black solid lines, respectively) on DH10–P medium supplemented with 0.5 mM of EtPhn (a) or AePhn (b) or under the same conditions but in continuous dark (dotted lines). As a negative control Syn OS-B′ and Syn OS-A cells were grown in DH10–P medium in the light (−P, triangles dotted gray lines). The graphs show the means and standard deviations (error bars) of measurements taken from biological triplicates. Cells per ml are shown on a log scale. The color reproduction of this figure is available on the html full text version of the manuscript.
Putative Phnases
These results suggest that Syn OS-A and Syn OS-B′ may have multiple pathways for Phn acquisition and assimilation. In addition to C–P lyases, bacterial Phnases, such as phosphonoacetaldehyde phosphonohydrolase (phnA) and phosphonopyruvate hydrolase, can cleave the C–P bond (Quinn et al., 2007; White and Metcalf, 2007). Phnases belong to the haloacid dehalogenases (HAD) superfamily (Interpro IPR006439), phosphoesterases, ATPases, phosphomutases, dehalogenases and Phnases, which exert their effect on a diverse set of substrates. The HAD domain can be recognized by certain key conserved features such as conserved aspartic acid (Asp) residues that are involved in the phosphotransfer reaction. The Rossman fold that comprises the core in which the phosphotransfer occurs is formed by four loops that contain ‘core residues’ conserved throughout the HAD superfamily. The conservation of these residues within short motifs and the additions of specific inserts called ‘caps’ are potential means of identification of specific members of the family (Allen and Dunaway-Mariano, 2004; Lahiri et al., 2004; Burroughs et al., 2006) Despite these general identifying features, Phnases are not very well characterized, so identification based on homology is not definitive.
BLAST searches using the amino-acid sequence encoded by the phnX gene (phosphonoacetaldehyde phosphonohydrolase, EC 3.11.1.1) of Bacillus cereus AH820 (YP_002450365), which is well characterized at both the catalytic and structural levels, identified at least three putative Phnase-encoding genes on the genomes of Syn OS-A and Syn OS-B′ (Lahiri et al., 2004). These are CYA_1475 (ortholog is CYB_1669, 74% AAID), CYA_2058 (ortholog is CYB_0748, 90% AAID) and CYA_2485 (ortholog is CYB_2092, 98% AAID). Alignment of PhnX from B. cereus and Pseudomonas aeruginosa with the putative Phnases of Syn OS-A and Syn OS- B′ (Supplementary Figure S2) suggests that these may all function as Phnases, but without further biochemical characterization their specificity cannot be easily established.
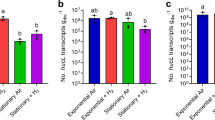
We estimated the transcript abundance of the putative Phnases under conditions of P starvation, as well as in cultures in which MePhn, EtPhn or AePhn were the sole P source. Transcripts from two putative Phnases (CYA_1475 and CYA_2058) increased in abundance (from ∼75- to 225-fold) when cells were grown in medium lacking Pi (2 weeks) and supplemented with either EtPhn or AePhn (MePhn did not elicit accumulation of these transcripts). As expected, the absence of P was not sufficient to elicit accumulation of the putative Phnase transcripts (Figure 4). None of the conditions used elicited a significant change in the level of the CYA_2485 transcript. Furthermore, the CYA_1475 and CYA_2058 transcripts also increased in abundance in cells grown in medium containing Pi and either EtPhn or AePhn, indicating that transcription from the putative Phnase genes is controlled by the presence of the substrate, and is not strongly impacted by the Pi concentration of the medium. Consistent with this result, none of the putative Phnases has a predicted upstream consensus Pho box sequence that has recently been characterized for many cyanobacteria including Syn OS-B′ and Syn OS-A (Su et al., 2007). None of the Syn OS-B′ transcripts encoding putative Phnases showed a marked increase in cells grown in medium devoid of P or supplemented with MePhn, EtPhn or AePhn as sole sources of P (Figure 4).
Quantification of transcripts encoding putative Phnases under various growth conditions. Transcripts levels from CYA_1475, CYA_2058 and CYA_2485 of Syn OS-A (a) and from CYB_1669, CYB_2092 and CYB_0748 of Syn OS-B′ (b) after growth on DH10 medium (Pi), DH10–P medium (P starved) or DH10–P medium supplemented with MePhn, EtPhn or AePhn and DH10+P supplemented with MePhn. Samples were taken in exponential phase. qRT-PCR results (relative units, RU) show the means and standard deviations for data from three technical replicates, although similar results were obtained for biological replicates.
In situ expression of the key putative Phn utilization genes
We measured the abundance of transcripts encoding the C–P lyase of Syn OS-B′ and the Phnases of Syn OS-A in mat samples collected from Octopus Spring at different times of the diel cycle on 28–29 July 2007. qRT-PCR with specific primers was used to evaluate phnC (gene in C–P lyase operon of Syn OS-B′) and the putative Phnase (CYA_1475 and CYA_2058) transcript levels of Syn OS-A. The phnC transcript was highest in the evening, when irradiance levels were low and the mat was anoxic (Figure 5), with a subsequent decline in the transcript level over the course of the night. This transcript may be controlled by oxic conditions and/or the circadian program. The levels of transcripts for the Syn OS-A putative Phnases are highest at mid afternoon when the mat is still oxic, and then decline. Expression of putative Phnases of Syn OS-B′ was significantly lower than for the putative orthologs in Syn OS-A, and there was no clear trend in the pattern of expression (Supplementary Table S3).
In situ quantification of transcripts from putative Phnases (CYA_1475, CYA_2058) and PhnC over the diel cycle. Oxygen production is shown as the dotted curve; oxic (light) and anoxic (dark) state of the mat indicated by gray and black bars, respectively. Transcript levels (relative units, RU) of genes encoding the Phn transporter (PhnC, CYB_0159, diamonds) and the putative Phnases from Syn OS-A (CYA_1475, squares, CYA_2058, triangles) are shown. The color reproduction of this figure is available on the html full text version of the manuscript.
Discussion
Microorganisms in the environment are capable of synthesizing, taking up and using Phns (Kononova and Nesmeyanova, 2002; Quinn et al., 2007; White and Metcalf, 2007; Metcalf and van der Donk, 2009; Singh, 2009). Pathways for Phn biosynthesis are less well-studied than those for its degradation (Quinn et al., 2007) and analysis of genomic and metagenome data available from the hot springs (Bhaya et al., 2007) did not provide compelling evidence that Phn biosynthetic pathways are present in the microbial mat community. However, we did demonstrate that Syn OS-B′ can grow well in medium in which the only source of P is MePhn, EtPhn or AePhn, contingent on an acclimation period of approximately 3 weeks in the presence of Phn compounds (Adams et al., 2008). Over the initial period of acclimation, the presence of Phn in the medium suppressed the growth of the cells on Pi, suggesting that Phns may function as an antimetabolites and block certain cellular activities (Kononova and Nesmeyanova, 2002).
Syn OS-B′ can use MePhn or EtPhn as a sole P source in the light, and as a consequence cells release either methane or ethane. These results suggest that in the light, Syn OS-B′ cells take up Phns, which is then further metabolized by the cytoplasmic C–P lyase. Under these conditions, released Pi is assimilated whereas some of the reduced carbon moiety of the Phn is released into the atmosphere as methane or ethane. However, in the dark, the reduced C moiety of the Phn is integrated into the metabolic circuitry of the cell, may be through conversion to acetyl CoA. In other systems that have been studied, biodegradation appears to involve ribosylated organophosphonate intermediates (Avila et al., 1991; Hove-Jensen et al., 2010) and possibly result in the generation of more than one reduced C species. Transcripts encoding Phn transporter/C–P lyase subunits increase when Syn OS-B′ cells are starved for P (in the absence or presence of MePhn, EtPhn or AePhn), whereas the Phnase transcripts are not. Therefore, it is most likely that the Phn transporter/C–P lyase system is important for Phn use in Syn OS-B′ under the conditions used in this study (although we cannot eliminate the possibility that some Phn use is a consequence of Phnases). The finding that little, if any, of the organic C is released as a volatile compound in the dark, and that cells grow in the dark when the only reduced C is in the form of Phn, strongly indicates that Syn OS-B′ can exploit the energetic component of Phn at a time when it is limited in its ability to generate chemical bond energy through either photosynthesis or respiratory metabolism. Indeed, the Phn transport/C–P lyase system would allow Syn OS-B′ cells to transport the entire Phn molecule into the cytoplasm, where the C–P bond would be cleaved. The C backbones released could be integrated into assimilation pathways through the activities of enzymes such as acetyl-CoA synthetase (CYB_0240, CYA_0472) and ATP citrate lyase (CYB_1908, CYA_1739).
In contrast to Syn OS-B′, Syn OS-A maintained in medium containing MePhn, EtPhn or AePhn did not emit methane or ethane either in the light or dark and was unable to use any of these Phn compounds as a sole C source for growth in the dark. Apparently, Syn OS-A can access the P moiety of Phn, but cannot metabolize the reduced C moiety of the molecule. Syn OS-A appears to use various Phnases and two genes encoding putative Phnases are induced during exposure of Syn OS-A to specific Phn substrates. In Salmonella sp. Phnase is induced under P starvation (Jiang et al., 1995) but in Rhizobium huakuii PMY1, Phnase are not under control of the Pho regulon. The ability of Syn OS-A to induce specific Phnases, in the presence of P, might indicate that Phns are available in these environments. However, information regarding the source, levels and use of P sources, including Phns, in many environments, including hot springs is limited and suffers from technical challenges (Stauffer and Thompson, 1978; Stauffer, 1982; Papke et al., 2003).
Some Phnases may be membrane associated potentially accessing the extracellular environment (Mendz et al., 2005), as a consequence, Pi may be generated in the extracellular space and then rapidly taken up and assimilated by the cells. If so, Syn OS-A might be unable to use EtPhn or AePhn as a sole source of C because it cannot take up the extracellular C compound released during Phn catalysis. Phnase transcripts from CYA_1475 and CYA_2058 accumulate in the presence of EtPhn and AePhn, but not MePhn, and yet Syn OS-A can use all of these compounds as P sources. These results suggest that other as yet unidentified Phnases may be involved in the use of MePhn by Syn OS-A, and that substrate specificity may be associated with each of the putative Phnases (Kononova and Nesmeyanova, 2002; White and Metcalf, 2007). There may be other novel mechanisms by which Phns are degraded by microorganisms (Fox and Mendz, 2006; Quinn et al., 2007).
Recently, Karl et al. (2008) have shown that microbial Phn degradation results in methane generation in aerobic marine environments, providing evidence for ‘unconventional’ methanogenesis. This bacterial catabolism of Phns might explain why oceanic methane concentrations vastly exceed the levels predicted by basic biological and chemical principles (Ingall, 2008). The ability of Trichodesmium sp. to metabolize as well as produce Phn compounds may explain why this cyanobacterium is so successful in low-P environments (Dyhrman and Haley, 2006; Dyhrman et al., 2007, 2009). Marine unicellular cyanobacteria contain putative Phn transporters and Phnases (Tetu et al., 2009). Marine bacteria including Vibro sp. and Planctomyces sp. have the ability to grow on Phns (Dyhrman et al., 2007). Moreover, the widespread distribution of the phnA gene, which encodes a phosphonoacetate hydrolase, underscores the potential importance of phosphonates in marine ecosystems, including corals (Gilbert et al., 2009; Martinez et al., 2009; Thomas et al., 2010).
Methane production in low-sulfate, hot-spring algal–bacterial mats has been ascribed to methanogenic bacteria as well as eukaryotic organisms (Ward, 1978). Here we show that the Phn transporter/C–P lyase genes and putative Phnase genes are expressed in situ, so cyanobacteria could produce methane as a by-product of Phn metabolism (levels of Phns in these environments have not been measured). Transcript profiles suggest that the Phn transporter/C–P lyase genes are expressed when the mat is anoxic, suggesting that the level of the Phn transporter/C–P lyase is higher at night. In contrast, Phnase genes are expressed in mid afternoon when the cells are photosynthesizing and are not likely to require additional fixed C. Hence, Phn cleavage, possibly in the extracellular environment during the day, would allow cells to capture the P in the molecule whereas the fixed C moiety would be released into the environment where it could be exploited by heterotrophs in the mat. These results highlight the ability of terrestrial cyanobacteria to use multiple systems to acquire and metabolize Phns from the environment, but investigations to elucidate the precise role of these specific activities in shaping population dynamics and micro-niche habitation are only just beginning.
References
Adams MM, Gomez-Garcia MR, Grossman AR, Bhaya D . (2008). Phosphorus deprivation responses and phosphonate utilization in a thermophilic Synechococcus sp. from microbial mats. J Bacteriol 190: 8171–8184.
Allen KN, Dunaway-Mariano D . (2004). Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem Sci 29: 495–503.
Allewalt JP, Bateson MM, Revsbech NP, Slack K, Ward DM . (2006). Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the Octopus Spring microbial mat community of Yellowstone National Park. Appl Environ Microbiol 72: 544–550.
Avila LZ, Draths KM, Frost JW . (1991). Metabolites associated with organophosphonate C–P bond cleavage: chemical synthesis and microbial degradation of [32P]-ethylphosphonic acid. Bioorgan Med Chem 1: 51–54.
Benitez-Nelson CR, O’Neill L, Kolowith LC, Pellecia P, Thunell R . (2004). Phosphonates and particulate organic phosphorus cycling in an anoxic marine basin. Limnol Oceanogr 49: 1593–1604.
Bhaya D, Grossman AR, Steunou AS, Khuri N, Cohan FM, Hamamura N et al. (2007). Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J 1: 703–713.
Burroughs AM, Allen KN, Dunaway-Mariano D, Aravind L . (2006). Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J Mol Biol 361: 1003–1034.
Clark LL, Ingall ED, Benner R . (1998). Marine phosphorus is selectively remineralized. Nature 393: 426.
Cook AM, Daughton CG, Alexander M . (1978). Phosphonate utilization by bacteria. J Bacteriol 133: 85–90.
Dyhrman ST, Ammerman JW, Van Mooy BAS . (2007). Microbes and the marine phosphorus cycle. Oceanography 20: 110–116.
Dyhrman ST, Benitez-Nelson CR, Orchard ED, Haley ST, Pellechia PJ . (2009). A microbial source of phosphonates in oligotrophic marine systems. Nat Geosci 2: 696–699.
Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB et al. (2006). Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439: 68–71.
Dyhrman ST, Haley ST . (2006). Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl Environ Microbiol 72: 1452–1458.
Elser JJ, Matthew ES, Bracken E, Cleland E, Daniel S, Gruner W et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10: 1135–1142.
Falkowski PG, Fenchel T, Delong EF . (2008). The microbial engines that drive Earth's biogeochemical cycles. Science 320: 1034–1039.
Forlani G, Pavan M, Gramek M, Kafarski P, Lipok J . (2008). Biochemical bases for a widespread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant Cell Physiol 49: 443–456.
Fox EA, Mendz GL . (2006). Phosphonate degradation in microorganisms. Enzyme Microb Technol 40: 145–150.
Gilbert JA, Thomas S, Cooley NA, Kulakova A, Field D, Booth T et al. (2009). Potential for phosphonoacetate utilization by marine bacteria in temperate coastal waters. Environ Microbiol 11: 111–125.
Hildebrand RL, Henderson TG . (1983). Phosphonic acids in nature. In Hildebrand, RL (ed.). The Role of Phosphonates in Living Systems. CRC Press: Boca Raton, FL.
Hove-Jensen B, Rosenkrantz TJ, Zechel DL, Willemoes M . (2010). Accumulation of intermediates of the carbon–phosphorus lyase pathway for phosphonate degradation in phn mutants of Escherichia coli. J Bacteriol 192: 370–374.
Ilikchyan IN, McKay RM, Zehr JP, Dyhrman ST, Bullerjahn GS . (2009). Detection and expression of the phosphonate transporter gene phnD in marine and freshwater picocyanobacteria. Environ Microbiol 11: 1314–1324.
Ingall ED . (2008). Making methane. Nat Geoscience 1: 419–420.
Iqbal S, Parker G, Davidson H, Moslehi-Rahmani E, Robson RL . (2004). Reversible phase variation in the phnE gene, which is required for phosphonate metabolism in Escherichia coli K-12. J Bacteriol 186: 6118–6123.
Jiang W, Metcalf WW, Lee KS, Wanner BL . (1995). Molecular cloning, mapping, and regulation of Pho regulon genes for phosphonate breakdown by the phosphonatase pathway of Salmonella typhimurium LT2. J Bacteriol 177: 6411–6421.
Karl DM, Beversdorf L, Björkman KM, Church MJ, Martinez A, Delong EF . (2008). Aerobic production of methane in the sea. Nat Geosci 1: 473–478.
Kilian O, Steunou AS, Fazeli F, Bailey S, Bhaya D, Grossman AR . (2007). Responses of a thermophilic Synechococcus isolate from the microbial mat of Octopus Spring to light. Appl Environ Microbiol 73: 4268–4278.
Kolowith LC, Ingall ED, Benner R . (2001). Composition and cycling of marine organic phosphorus. Limnol Oceanogr 46: 309–320.
Kononova SV, Nesmeyanova MA . (2002). Phosphonates and their degradation by microorganisms. Biochemistry (Mosc) 67: 184–195, 184–195.
Lahiri SD, Zhang G, Dai J, Dunaway-Mariano D, Allen KN . (2004). Analysis of the substrate specificity loop of the HAD superfamily cap domain. Biochemistry 43: 2812–2820.
Martinez A, Tyson GW, Delong EF . (2009). Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ Microbiol 12: 222–238.
Mendz GL, Megraud F, Korolik V . (2005). Phosphonate catabolism by Campylobacter spp. Arch Microbiol 183: 113–120.
Metcalf WW, van der Donk WA . (2009). Biosynthesis of phosphonic and phosphinic acid natural products. Annu Rev Biochem 78: 65–94.
Mills MM, Ridame C, Davey M, La Roche J, Geider RJ . (2004). Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429: 292–294.
Nowack B . (2003). Environmental chemistry of phosphonates. Water Res 37: 2533–2546.
Orchard ED, Webb EA, Dyhrman ST . (2009). Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ Microbiol 11: 2400–2411.
Papke RT, Ramsing NB, Bateson MM, Ward DM . (2003). Geographical isolation in hot spring cyanobacteria. Environ Microbiol 5: 650–659.
Quinn JP, Kulakova AN, Cooley NA, McGrath JW . (2007). New ways to break an old bond: the bacterial carbon–phosphorus hydrolases and their role in biogeochemical phosphorus cycling. Environ Microbiol 9: 2392–2400.
Rozen S, Skaletsky H . (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386.
Sanudo-Wilhelmy SA . (2006). Oceanography: a phosphate alternative. Nature 439: 25–26.
Singh BK . (2009). Organophosphorus-degrading bacteria: ecology and industrial applications. Nat Microbiol Rev 7: 156–164.
Stauffer RE . (1982). Fluorapatite and fluorite solubility controls on geothermal waters in Yellowstone National Park. Geochemica Cosmochemica Acta 46: 465–474.
Stauffer RE, Thompson JM . (1978). Phosphorous in hydrothermal waters of Yellowstone National Park, Wyoming. J Res US Geol Surv 6: 755–763.
Steunou AS, Bhaya D, Bateson MM, Melendrez MC, Ward DM, Brecht E et al. (2006). In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc Natl Acad Sci USA 103: 2398–2403.
Steunou AS, Jensen SI, Brecht E, Becraft ED, Bateson MM, Kilian O et al. (2008). Regulation of nif gene expression and the energetics of N2 fixation over the diel cycle in a hot spring microbial mat. ISME J 2: 364–378.
Su Z, Olman V, Xu Y . (2007). Computational prediction of Pho regulons in cyanobacteria. BMC Genomics 8: 156–167.
Ternan NG, Mc Grath JW, Mc Mullan G, Quinn JP . (1998). Organophosphonates: occurrence, synthesis and biodegradation by microorganisms. World J Microbiol Biotech 14: 635–647.
Tetu SG, Brahamsha B, Johnson DA, Tai V, Phillippy K, Palenik B et al. (2009). Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. ISME J 3: 835–849.
Thomas S, Burdett H, Temperton B, Wick R, Snelling D, McGrath JW et al. (2010). Evidence for phosphonate usage in the coral holobiont. ISME J 4: 459–461.
Torriani-Gorini A, Yagil E, Simon S . (1994). Phosphate in Microorganisms: Cellular and Molecular Biology. ASM Press: Washington, DC.
Van Mooy BA, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblízek M et al. (2009). Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458: 69–72.
Ward DM . (1978). Thermophilic methanogenesis in a hot-spring algal–bacterial mat (71 to 30 degrees C). Appl Environ Microbiol 35: 1019–1026.
Whelan JA, Russell NB, Whelan MA . (2003). A method for the absolute quantification of cDNA using real-time PCR. J Immunol Methods 278: 261–269.
White AK, Metcalf WW . (2007). Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61: 379–400.
Wu J, Sunda W, Boyle EA, Karl DM . (2000). Phosphate depletion in the western North Atlantic Ocean. Science 289: 759–762.
Yakovleva GM, Kim SK, Wanner BL . (1998). Phosphate-independent expression of the carbon–phosphorus lyase activity of Escherichia coli. Appl Microbiol Biotechnol 49: 573–578.
Acknowledgements
This study was supported by the National Science Foundation (EF-0328698), Stanford University (MD), Venture Grant from Colorado College (MB-H) and the Carnegie Institution (DB, MG). We thank George Asimenos for primer design, and Melissa Adams and Fariba Fazeli for expert technical assistance; Blaise Hamel and Sheila I Jensen for collecting and processing samples; the US National Park Service and personnel from Yellowstone National Park.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Gomez-Garcia, M., Davison, M., Blain-Hartnung, M. et al. Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats. ISME J 5, 141–149 (2011). https://doi.org/10.1038/ismej.2010.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.96
Keywords
This article is cited by
-
Phosphonate consumers potentially contributing to methane production in Brazilian soda lakes
Extremophiles (2024)
-
Marine picocyanobacterial PhnD1 shows specificity for various phosphorus sources but likely represents a constitutive inorganic phosphate transporter
The ISME Journal (2023)
-
Carbon-Phosphorus Lyase—the State of the Art
Applied Biochemistry and Biotechnology (2020)
-
Strains of the toxic and bloom-forming Nodularia spumigena (cyanobacteria) can degrade methylphosphonate and release methane
The ISME Journal (2018)
-
Glyphosate dose modulates the uptake of inorganic phosphate by freshwater cyanobacteria
Journal of Applied Phycology (2018)