Abstract
The fungus Aspergillus sydowii is the causative agent of epidemics that affect gorgonian corals (sea fans) and has significantly affected their populations in the Caribbean Sea. We have isolated a strain of A. sydowii from healthy marine sponges (Spongia obscura) collected in Bahamian inshore waters. After its identification on the basis of morphology, molecular markers and chemical profiling followed by pathogenicity tests, we found this strain to be highly similar to a strain isolated from diseased coral, and have shown the capacity of this fungus to persist in sponge environment. Our findings suggest that sponges have the possibility of being reservoirs of a potential marine pathogen.
Similar content being viewed by others
Main
Microbial diseases of marine invertebrates have been rapidly increasing in recent decades (Rosenberg and Loya, 2004). A well-known example is the fungal epizootic infection of sea fan corals (Gorgonia ventalina) by strains of Aspergillus sydowii (Geiser et al., 1998) that spread throughout the Caribbean Sea during the 1990s (Smith et al., 1996). As many Aspergilli are opportunistic pathogens of stressed and immune-compromised hosts, sea fan aspergillosis may have been facilitated by the combination of environmental conditions (for example, increased hurricane incidence, wind and/or human-conveyed propagules; Garrison et al., 2003), with the presence of fungal strains that are subject to ongoing gene flow between different (and distant) geographic areas (Rypien et al., 2008). Fungi may also reside in the marine environment in latent forms on the host organism or on potential symptomless carriers.
Association of filamentous fungi with marine sponges was reported by Holler et al. (2000), but sponges' potential to serve as reservoirs for marine pathogens is yet to be shown. We have found that fungal association with sponges is a common phenomenon, having isolated over hundreds of filamentous fungal cultures from sponge samples collected from globally distant geographical locations (Yarden, Carmeli and Ilan, unpublished), yet the ecological role of these fungi is unknown.
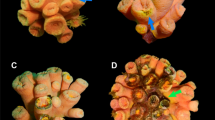
Fungal isolate 382, was isolated from healthy Spongia obsucra (Figure 1a) growing on mangrove roots in a pristine tidal channel on the East end of Grand Bahama Island. Small pieces of the sponge inner tissue were rinsed with sterile seawater and inoculated on KMV ((glucose (1 g l−1), gelatin hydrolysate (1 g l−1), yeast extract (0.1 g l−1), bacto peptone (0.1 g l−1), granulated agar (15 g l−1) and chloramphenicol (0.25 g l−1)) agar petri dishes. Emerging colonies were transferred to potato dextrose agar (Difco Laboratories, Detroit, MI, USA) and incubated at 25 °C to allow colony development.
(a) The marine sponge Spongia obscura. (b) Aspergillosis (necrotic rings) on the sea fan (Gorgonia ventalina). (c) Effect of different fungal inocula on Gorgonia ventalina. Strains of A. sydowii known to either be pathogenic on the sea fan (SA-25), non-pathogenic (NRRL249) or isolated during the course of this study from the marine sponge S. obscura (382) were used as inocula. A fungus-free gauze was used a control (NI). The extent of infected tissue was measured 5 days after inoculation. Bars indicate standard error.
Isolate 382 exhibited morphological and molecular phylogenetic characteristics of A. sydowii, the causative agent of sea fan aspergillosis (partial 18S rDNA and trpC sequences; submitted to GenBank under accession numbers DQ114468 and DQ104445, respectively). These sequences differ from those of an identical species isolated from terrestrial environments (Geiser et al., 1998). The metabolite secretion profile of this marine strain was also analyzed. Production fermentation was performed, for 10–14 days in fifteen 250 ml flasks containing seawater with potato dextrose broth. An ethyl acetate medium extract was fractionated by high performance liquid chromatography on an octadodecyl silica column and the structures of pure compounds were elucidated by one-dimensional (1H and 13C) and two-dimensional (homonuclear correlation spectroscopy, heteronuclear multiple quantum coherence, heteronuclear multiple bond coherence and rotating-frame overhauser effect spectroscopy) nuclear magnetic resonance experiments and mass spectrometry analyses. We determined that the strain produces sydonic and sydowic acids, 6-O-methyl-sydonic acid, 6-O-methyl-13-hydroxy-sydonic acid and diorcinol, metabolites that are characteristic of A. sydowii (Hamasaki et al., 1975, 1978). Inoculation experiments using healthy sea fans (Gorgonia ventalina from Linsey's Reef, San Salvador, Bahamas) were performed by gauze-infiltrated inocula (following Smith et al. (1996)), with three A. sydowii strains: 382, SA-25 (isolated from an infected sea fan from Saba; Smith et al., 1996) and NRRL 249 (nonpathogenic A. sydowii strain; Holler et al., 2000). Infection was assessed by measuring the radius of infected tissue from the point of inoculation (Figures 1b and c). To treat SA-25 and 382 strains, hyphae were observed microscopically extending into the sea fans' coenenchyme. Tissue damage from NRRL-249 strain and the control during the inoculation process accounted for the observed measurements of these treatments. Even though DNA-based taxonomy is becoming increasingly accurate (Geiser et al., 2007), the use of as much data from as many sources as possible in making taxonomic decisions, including secondary metabolism (a key feature of fungi), advantageous. The latter is also significant in the chemical ecology of sessile marine biota and the microorganisms that are associated with them. The combination of genetic data along with chemical profiling and pathogenicity tests strongly support our conclusion concerning the presence of a potential gorgonian coral pathogen in a marine sponge from a nearby inshore habitat.
The sampled sponges' distance from coral reefs (∼5 km) diminishes the likelihood that fungi were present within the sponge because of contact with, or physical proximity to, Aspergillus-harboring corals. We attribute the fungal presence to the sponge's high rate of water filtration and to the presence of perhaps minute quantities of fungal propagules originating from infected coral, external sources such as dust (Weir et al., 2004), the mangrove sediment, proximal terrestrial runoff, saprophytic presence in the eulittoral zone (Roth et al., 1964) or propagation within the sponge.
As marine sponges (including Spongia spp.) can be potentially hostile environments for fungi (Pettit, 1997), we examined whether strain 382 could persist in Spongia obscura. Conidia of strain 382 (4 × 107 per ml of sterile seawater) were placed into dialysis tubes (6.3 mm diameter, 10–12 000 Dalton cutoff Visking dialysis tubing; Medicell International, London UK). Two tubes were inserted into narrow incisions (4 cm deep into the sponge mesohyl) made in three specimens of S. obscura growing on submerged mangrove roots (50–100 cm below the water surface). Control tubes were placed on adjacent roots in submerged cages (allowing free seawater flow). Three days after inoculation, the number of viable colony-forming units was reduced by 50% in all samples, with no significant difference between sponge-inserted and control samples. In additional experiments, identical tubes with conidial suspensions were inserted into specimens of the sponge Ircinia strobilina (present, downstream, in the same tidal channel described above). In contrast to S. obscura, this species is also prevalent at greater depths and in the vicinity of gorgonian coral colonies. In this case, survival rates of A. sydowii were even higher (exceeding 80%). These results suggest that (i) within the tested time frame the sponge environment (be it S. obscura or I. strobilina) was not any more deleterious to the fungus than was the adjacent mangrove habitat and (ii) A. sydowii can persist in sponge species that inhabit both the mangrove habitat, as well as the reef tract in close proximity to the potential host. To determine if fungal conidia were readily taken up by sponge filtration, and whether viable conidia could persist after natural filtration and contact with the sponge (and sponge-associated microorganisms), we exposed two S. obscura sponges to conidial suspensions (lacking the tube barrier described above). A conidial suspension (5 × 108 in 50 ml sterile seawater) was injected into a 2 l plastic bag enclosing each sponge for 20 min. Sponges were sampled periodically by isolating a 1-cm3 fragment of inner tissue that was subsequently rinsed with sterile seawater and macerated with mortar and pestle. Aliquots of the macerated sponge were diluted in sterile seawater and plated on potato dextrose agar amended with chlormaphenicol. A total of 40±10 A. sydowii colony-forming units were isolated from the inoculated samples 28 h after exposure. Similar colony-forming unit numbers were obtained when the same sponges were sampled 9 days later, showing the viability and sustainability of the fungus within the sponge, without notably affecting the sponge health (control sponges yielded only several colonies of various fungal species). These results indicate that this sponge can both take up and retain viable conidia of A. sydowii.
Although fungi can be readily isolated from marine sponges, their role in the sponge diet, or as part of the sponge microbiota, remains to be elucidated. Here, we have shown that in addition to the potential substrates in the marine environment on which A. sydowii (and other fungi) may reside as saprophytes (Roth et al., 1964), a live sponge may be a symptomless carrier of a marine pathogen of gorgonian coral. It remains to be elucidated whether the possible mechanism(s) involved in the function of such a potential disease carrier includes the release of fungal propagules after fungal growth and conidiation (or fragmentation within the living sponge), or after the sponges' death. It is tempting to speculate that the presence of sponges along a potential route between terrestrial habitats and potential hosts located on the reef tract provides the possibility of pathogen retention by the natural filter feeders and eventual ‘sponge hopping’ as a mechanism of fungal propagule survival and dispersal in the aquatic environment.
References
Garrison VH, Shinn EA, Foreman WT, Griffin DW, Holmes CW, Kellogg CA et al. (2003). African and Asian dust: from desert soils to coral reefs. Bioscience 53: 469–480.
Geiser DM, Klich MA, Frisvad JC, Peterson SW, Varga J, Samson RA . (2007). The current status of species recognition and identification in Aspergillus. Stud Mycol 59: 1–10.
Geiser DM, Taylor JW, Ritchie KB, Smith GW . (1998). Cause of sea-fan death in the West Indies. Nature 394: 137–138.
Hamasaki T, Nagayama K, Hatsuda Y . (1978). Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric Biol Chem 42: 37–40.
Hamasaki T, Sato Y, Hatsuda Y . (1975). Isolation of new metabolites from Aspergillus sydowi and structure of sydowic acid. Agric Biol Chem 39: 2337–2340.
Holler U, Wright A, Matthee G, Konig GM, Draeger S, Aust H-J et al. (2000). Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol Res 104: 1354–1365.
Pettit GR . (1997). Antifungal activity of the spongistatins. United States Patent 5883120.
Rosenberg E, Loya Y . (2004). Coral Health and Disease. Springer-Verlag: New York, USA.
Roth FJ, Orpurt PA, Ahearn DG . (1964). Occurance and distribution of fungi in a subtropical marine environment. Can J Bot 42: 375–383.
Rypien KL, Andras JP, Harvell CD . (2008). Globally panmictic population structure in the opportunistic fungal pathogen Aspergillus sydowii. Mol Ecol 17: 4068–4078.
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB . (1996). Caribbean sea-fan mortalities. Nature 383: 487.
Weir JR, Garrison V, Smith GW, Shinn EA . (2004). The relationship between gorgonian coral (Cnidaria: Gorgonacea) disease and African dust storms. Aerobiologia 20: 119–126.
Acknowledgements
Funding was provided by the National Science Foundation, Biological Oceanography Program (to JRP, OCE-0095724 and 0550468, including UNOLS ship support on the RV Seward Johnson), by the US–Israel Binational Science Foundation (BSF 200-321) and the Israel Science Foundation. We thank the government of the Bahamas for permission to perform research in their territorial waters.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ein-Gil, N., Ilan, M., Carmeli, S. et al. Presence of Aspergillus sydowii, a pathogen of gorgonian sea fans in the marine sponge Spongia obscura. ISME J 3, 752–755 (2009). https://doi.org/10.1038/ismej.2009.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2009.18
Keywords
This article is cited by
-
Identification of a novel anthocyanin synthesis pathway in the fungus Aspergillus sydowii H-1
BMC Genomics (2020)
-
Onychomycosis Associated with Superficial Skin Infection Due to Aspergillus sydowii in an Immunocompromised Patient
Mycopathologia (2019)
-
Introduced ascidians harbor highly diverse and host-specific symbiotic microbial assemblages
Scientific Reports (2017)
-
Diversity of fungi isolated from three temperate ascidians
Symbiosis (2015)
-
Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress
The ISME Journal (2013)