Abstract
There is considerable interest in understanding the biological mechanisms that regulate carbon exchanges between the land and atmosphere, and how these exchanges respond to climate change. An understanding of soil microbial ecology is central to our ability to assess terrestrial carbon cycle–climate feedbacks, but the complexity of the soil microbial community and the many ways that it can be affected by climate and other global changes hampers our ability to draw firm conclusions on this topic. In this paper, we argue that to understand the potential negative and positive contributions of soil microbes to land–atmosphere carbon exchange and global warming requires explicit consideration of both direct and indirect impacts of climate change on microorganisms. Moreover, we argue that this requires consideration of complex interactions and feedbacks that occur between microbes, plants and their physical environment in the context of climate change, and the influence of other global changes which have the capacity to amplify climate-driven effects on soil microbes. Overall, we emphasize the urgent need for greater understanding of how soil microbial ecology contributes to land–atmosphere carbon exchange in the context of climate change, and identify some challenges for the future. In particular, we highlight the need for a multifactor experimental approach to understand how soil microbes and their activities respond to climate change and consequences for carbon cycle feedbacks.
Similar content being viewed by others
Introduction
Ongoing global climate change caused by human-induced increases in greenhouse gases represents one of the biggest scientific and political challenges of the 21st century. Of these, perhaps the greatest is the need to better understand the biological mechanisms regulating carbon exchanges between the land, oceans and atmosphere and how these exchanges will respond to climate change through climate–ecosystem feedbacks, which could amplify or dampen regional and global climate change (Heimann and Reichstein, 2008). Terrestrial ecosystems play a major role in such climate-feedbacks because they release and absorb greenhouse gases, such as carbon dioxide, methane and nitrous oxides, while storing large quantities of carbon in living vegetation and soils, thereby acting as a significant global carbon sink (Schimel et al., 1994). Many interacting factors affect the sink activity of terrestrial ecosystems, including natural and anthropogenic disturbances (Magnani et al., 2007), agricultural land use (Smith et al., 2008), nitrogen (N) enrichment (Beedlow et al., 2004), sulphur deposition (Monteith et al., 2007) and changes in atmosphere ozone concentration (Sitch et al., 2007). The influence of climate change on the soil carbon sink remains a major area of uncertainty, especially as there is scope for warming to increase the liberation of carbon dioxide from soil to atmosphere due to enhanced microbial breakdown of soil organic matter. Such acceleration in carbon loss from soil could significantly exacerbate the soil carbon cycle feedback if predicted climate change scenarios are correct (Cox et al., 2000; Friedlingstein et al., 2006).
Ultimately, the net effect of climate change on ecosystem carbon budgets depends on the balance between photosynthesis and respiration (that is, autotrophic root respiration and heterotrophic soil microbial respiration). While our knowledge of the assimilatory component (that is photosynthesis) of the carbon cycle and its response to climate change is well advanced (Bahn et al., 2008), there are considerable gaps in our understanding of the response of soil respiration (Trumbore, 2006). This lack of understanding of soil respiration and its sensitivity to climate change stems from the fact that it is regulated by a myriad of factors including complex interactions and feedbacks between climate, plants, their herbivores and symbionts and free-living heterotrophic soil microbes (Wardle et al., 2004; Högberg and Read, 2006; De Deyn et al., 2008). The issue is complicated further by the knowledge that soil microbes act as important determinants of plant community diversity and productivity (Wardle et al., 2004; van der Heijden et al., 2008) and hence the quality and quantity of carbon input to soil (De Deyn et al., 2008). In this paper, we argue that to understand the potential negative and positive contributions of soil microbes to global warming requires explicit consideration of both direct and indirect impacts of climate change on soil microorganisms and the capacity for feedback to greenhouse gas production. We illustrate this by examining the role that microbial feedbacks play in regulating soil land–atmosphere carbon exchange, and, in doing so, emphasize the urgent need for greater understanding of how soil microbial ecology contributes to climate change.
Soil microbes, ecosystem carbon exchange and climate change
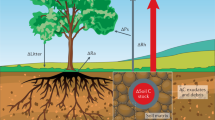
There are a myriad of ways that soil microbes and their metabolic activity can influence land–atmosphere carbon exchanges, but these can broadly be divided into those that affect ecosystem carbon dioxide and methane uptake, and those that control carbon loss from soil through respiration and methane production (Figure 1). Importantly, climate change has both direct and indirect effects on the activities of soil microbes that feedback greenhouse gases to the atmosphere and contribute to global warming: direct effects include the influence on soil microbes and greenhouse gas production of temperature, changing precipitation and extreme climatic events, whereas indirect effects result from climate-driven changes in plant productivity and diversity which alter soil physicochemical conditions, the supply of carbon to soil and the structure and activity of microbial communities involved in decomposition processes and carbon release from soil. Here, we use direct and indirect effects of climate change as a framework to illustrate the role of soil microbes and microbial metabolism in carbon cycle feedbacks and the consequences for climate change.
Direct and indirect effects of climate change on soil microbial communities and routes of feedback to global warming through carbon dioxide production. Direct effects include the influence on soil microbes and greenhouse gas production of temperature, changing precipitation and extreme climatic events, whereas indirect effects result from climate-driven changes in plant productivity and vegetation structure which alter soil physicochemical conditions, the supply of carbon to soil and the structure and activity of microbial communities involved in decomposition processes and carbon release from soil. Background image courtesy of Jill Colquhoun Bardgett.
Direct climate-microbe feedbacks
One of the most widely discussed contributions of soil microbes to climate change is their role in soil organic matter decomposition and the notion that global warming will accelerate rates of heterotrophic microbial activity, thereby increasing the efflux of CO2 to the atmosphere and exports of dissolved organic carbon by hydrologic leaching (Jenkinson et al., 1991; Davidson and Janssens, 2006). Because rates of soil respiration are thought to be more sensitive to temperature than primary production (Jenkinson et al., 1991; Schimel et al., 1994), it is predicted that climate warming will increase the net transfer of carbon from soil to atmosphere, thereby creating a positive feedback on climate change (Cox et al., 2002). While it is well established that temperature is an important determinant of rates of organic matter decomposition, the nature of the relationship between temperature and heterotrophic microbial respiration and its potential to feedback to climate change, are far from clear (Davidson and Janssens, 2006; Trumbone, 2006).
A prime cause of this uncertainty is the inherent complexity and diversity of soil organic matter and the likelihood that the temperature dependence of microbial decomposition of soil carbon compounds of differing chemical composition and substrate quality will vary (Davidson and Janssens, 2006). For example, there is evidence that the temperature sensitivity of litter decomposition increases as the quality of organic carbon consumed by microbes declines (Fierer et al., 2005), which is consistent with kinetic theory which indicates greater temperature sensitivity for decomposition of recalcitrant carbon pools (Knorr et al., 2005). However, there is still much uncertainty on this subject. For example, other studies suggest that the temperature sensitivity of more recalcitrant substrates is similar (Fang et al., 2005; Conen et al., 2006) or less than (Luo et al., 2001; Melillo et al., 2002; Rey and Jarvis, 2006) that of more labile substrates, while a recent synthesis of new and previously published soil C incubation data showed that temperature sensitivity of relatively more resistant organic matter is greater than that of relatively more labile substrates (Conant et al., 2008). There is also considerable potential for various environmental constraints, such as physical and chemical protection of organic matter, to decrease substrate availability for microbial attack, thereby dampening microbial responses to warming (Davidson and Janssens, 2006). The picture is further complicated by uncertainty about how reactive different microbial groups and species are to temperature change (Kandeler et al., 1998; Bardgett et al., 1999), and whether short-term increases in carbon mineralization—which are commonly observed in warming experiments in the field (Luo et al., 2001; Melillo et al., 2002)—will be sustained due to depletion of substrate availability and acclimation of soil microbial communities to higher temperature (Kirschbaum, 2004). This microbial process level uncertainty extends to unreliable model predictions of soil carbon feedbacks to climate change (Kirschbaum, 2006), and resolving this issue represents a major research challenge for the future.
It is likely that climate change-related increases in the frequency of extreme weather events, such as drought and freezing, will have even greater effects on microbes and their activities than overall changes in temperature and precipitation. It is well established that both drought and freezing can have substantial direct effects on microbial physiology and the composition of the active microbial community, with important consequences for ecosystem-level carbon and nutrient flows (Schimel et al., 2007). However, effects of these stressors on microbial communities and consequences for carbon exchange are likely to vary substantially across ecosystems. Increased frequency and intensity of drought in drier ecosystems, for example, may result in moisture-limiting conditions for microbial activity, creating a negative feedback on microbial decomposition and soil carbon loss as microbial respiration. This view is supported by studies of forest ecosystems which report significant falls in litter phenol oxidase activity and isoenzyme diversity, and soil bacterial and fungal biomass during dry periods (Nardo et al., 2004; Krivtsov et al., 2006) and by a manipulation experiment in dry Californian annual grassland, where the addition of water increased soil phenol oxidase activity (Henry et al., 2005). Rewetting of dry soil can also lead to a pulse of CO2 through increased microbial mineralization of cytoplasmic solutes, and in the longer-term, by decreasing the total amount of soil organic matter that is physically protected within microaggregates (Fierer and Schimel, 2003). However, this response to rewetting is less pronounced in soils that are frequently exposed to natural drying and rewetting cycles (Birch, 1958; Fierer and Schimel, 2002). In contrast, increased drought and drying in wetlands and peatlands will create more favourable conditions for microbial activity, by lowering the water table and introducing oxygen into previously anaerobic soil. This has been shown to increase the activity of phenol oxidases (Freeman et al., 2004a; Zibilske and Bradford, 2007), which play a pivotal role in the breakdown of complex organic matter and the cycling of phenolic compounds that may interfere with extra-cellular enzymes (Benoit and Starkey, 1968; Albers et al., 2004). Through what has been described as the ‘enzymic latch mechanism’ (Freeman et al., 2001), changes in the activity of extracellular phenol oxidases may directly affect the retention of carbon in soil through the breakdown of otherwise highly recalcitrant organic matter and by releasing extracellular hydrolase enzymes from phenolic inhibition (Freeman et al., 2001, 2004a). Because peatlands and wetlands are one of the largest stocks of terrestrial carbon (Ward et al., 2007), such enhanced breakdown of recalcitrant organic matter under drying could have major implications for the global carbon cycle (Freeman et al., 2004a).
While the increase in O2 availability that accompanies drought promotes organic matter decomposition in wetlands and peatlands, thereby increasing CO2 release, opposing effects occur for methanogenic pathways, in that methane emissions are reduced. Water table depth is a strong predictor of methane emissions (Roulet and Moore, 1995), and while this is generally assumed to be due to toxic effects of O2, there is also evidence that methanogens are more sensitive to desiccation (Fetzer et al., 1993). Also, toxic effects on methanogens of oxidized products of denitrification have been noted (Kluber and Conrad, 1998), while net methane emissions are also suppressed under drought conditions by the action of methanotrophic bacteria (King, 1992; Freeman et al., 2002). While not a focus of this paper, it is important to note that drought also has marked effects on nitrous oxide (N2O) emission from soils—a potent greenhouse gas that is increasing in atmospheric concentrations at the rate of 0.2–0.3% per year (Houghton et al., 1996)—with responses depending on the severity of drought: modest summer drought scenarios may have little effect on soil N2O emissions, whereas more extreme drought can increase the rate of N2O emission substantially (Dowrick et al., 1999).
While overall changes in temperature are likely to have strong effects on microbial communities and decomposition processes in arctic and alpine regions, climate change-related reductions in snow cover will also be of high importance. It has been estimated that 25% of Earth's permafrost could thaw by 2100 due to climate warming, releasing considerable amounts of otherwise protected organic matter for microbial decomposition (Anisimov et al., 1999), thus creating a positive feedback on climate change (Davidson and Janssens, 2006). Also, because snow is an important insulator of soil biological processes, predicted reductions in snow cover in alpine and arctic regions will increase soil freezing, with consequences for root mortality, nutrient cycling and microbial processes of decomposition (Groffman et al., 2001; Bardgett et al., 2005). Strong microbial responses to freeze–thaw have been detected in several studies, including increased microbial activity and greenhouse gas emission (Christensen and Tiedje, 1990; Sharma et al., 2006), altered microbial substrate use (Schimel and Mikan, 2005) and the expression of denitrifying genes, which lead to the release of N2O gas (Sharma et al., 2006). However, a recent synthesis of literature concluded that while freeze–thaw events might induce gaseous and/or solute losses of N from soils that are relevant at an annual time scale, they have little effect or will even reduce soil C losses as compared with unfrozen conditions (Matzner and Borken, 2008). Also, recent studies in subalpine forest in Colorado indicate that reduced snow cover can suppress rates of soil respiration due to a unique and highly temperature-sensitive soil microbial community that occurs beneath snow (Monson et al., 2006); such responses could have substantial consequences for winter soil microbial activity, carbon storage and CO2 efflux in alpine and arctic regions.
Indirect climate–microbe feedbacks
Climate change can also have marked indirect effects on soil microbial communities and their activity—and hence the potential for microbial feedback to climate change—through its influence on plant growth and vegetation composition. Such plant-mediated indirect effects of climate change on soil microbes operate through a variety of mechanisms, with differing routes of feedback to climate change, but these can broadly be separated into two. The first mechanism concerns the indirect effects of rising atmospheric concentrations of carbon dioxide on soil microbes, through increased plant photosynthesis and transfer of photosynthate carbon to fine roots and mycorrhizal fungi (Johnson et al., 2005; Högberg and Read, 2006; Keel et al., 2006) and heterotrophic microbes (Zak et al., 1993; Bardgett et al., 2005). It is well established that elevated carbon dioxide increases plant photosynthesis and growth, especially under nutrient-rich conditions (Curtis and Wang 1998) and this in turn increases the flux of carbon to roots, their symbionts and heterotrophic microbes through root exudation of easily degradable sugars, organic acids and amino acids (Díaz et al., 1993; Zak et al., 1993). The consequences of increased carbon flux from roots to soil for microbial communities and carbon exchange are difficult to predict, because they will vary substantially with factors such as plant identity, soil food web interactions, soil fertility and a range of other ecosystem properties (Wardle 2002; Bardgett, 2005). But, some potential outcomes for soil microbes and carbon exchange include: (i) increases in soil carbon loss by respiration and in drainage waters as dissolved organic carbon due to stimulation of microbial abundance and activity, and enhanced mineralization of recent and old soil organic carbon (Zak et al., 1993; Freeman et al., 2004b; Heath et al., 2005), a phenomenon known as ‘priming’ (Fontaine and Barot 2005; Kuzyakov, 2006; Dijkstra and Cheng, 2007); (ii) stimulation of microbial biomass and immobilization of soil N, thereby limiting N availability to plants, creating a negative feedback that constrains future increases in plant growth and carbon transfer to soil (Díaz et al., 1993); (iii) increased plant-microbial competition for N, leading to reduced soil N availability and microbial activity and suppression of microbial decomposition and ultimately increased ecosystem carbon accumulation (Hu et al., 2001); (iv) increased growth of mycorrhizal fungi (Klironomos et al., 1997; Staddon et al., 2004)—which receive carbon in the form of photosynthate directly from the host plant and retain this carbon, controlling its release to the soil microbial community (Högberg and Read, 2006)—and selection for beneficial fungal strains that help their host plant meet increased nutrient demands (Gamper et al., 2005), leading to a possible positive feedback on plant growth and carbon assimilation and enhanced stabilization of soil organic carbon through promotion of soil aggregation (Rillig and Mummey, 2006; Six et al., 2006); and (v) changes in root exudation are known to play a potentially important role through the promotion of methanogenesis and hence carbon loss from soil as methane (Ström et al., 2005), but the mechanisms involved are poorly understood.
The second mechanism concerns indirect effects of climate change on microbes through shifts in the functional composition and diversity of vegetation, which occur over longer timescales of decades to centuries. It is well established that climate change, especially warming and altered precipitation regimes, has the potential to alter the distribution of plant species and functional groups at both local and global scales (Prentice et al., 1992; Woodward et al., 2004). For example, recent changes in precipitation patterns have markedly affected vegetation composition in tropical rainforest (Engelbrecht et al., 2007) and African savanna (Sankaran et al., 2005), and warming is leading to rapid replacement of Canadian tundra by boreal forest (Danby and Hik, 2007) and pan-arctic shrub encroachment in arctic tundra (Epstein et al., 2004). Such changes in vegetation composition can strongly regulate carbon exchange by affecting uptake of CO2 by photosynthesis and by modifying the soil physical environment, for example by changes in root architecture and rooting depth (Jackson et al., 1996). But, a key mechanism by which climate-driven shifts in vegetation composition influence microbes and their metabolism, and hence carbon cycle feedback, is through changes in the quality and quantity of organic matter entering the soil as plant litter.
Leaf litter quality is known to differ consistently across plant functional groups (Aerts and Chapin, 2000; Dorrepaal et al., 2005) and correlates strongly with rates of decomposition and hence heterotrophic respiration: slow-growing plants, such as evergreen shrubs, produce poor quality litter which is low in nutrients and rich in recalcitrant compounds, such as lignin and phenolic acids and hence decompose slowly due to retardation of microbial activity; whereas, fast-growing plants, such as graminoids and N-fixers, produce relatively high quality litter that is rich in nutrients and decomposes very rapidly due to promotion of microbial activity (Wardle, 2002). Therefore, climate-driven increases in the dominance of evergreen shrubs with recalcitrant litter, as is occurring in the arctic, could constitute a negative feedback on carbon exchange and global warming due to reduced heterotrophic respiration (Cornelissen et al., 2007). Whereas, increased dominance of legumes over grasses, which is common in grassland subject to elevated atmospheric carbon dioxide (Hanley et al., 2004; Ross et al., 2004), could induce a positive feedback on microbial activity and carbon mineralization due to enhanced soil nutrient availability and decomposition of nutrient rich litter. As noted above, ecosystem-level shifts in substrate quality could have implications for the temperature sensitivity of decomposition and hence complicate further our ability to predict the magnitude of carbon cycle feedbacks (Fierer et al., 2005).
Plant functional groups also differ markedly in their mycorrhizal status (Read et al., 2004) and their mechanism for nutrient uptake, including acquisition of different chemical forms of nitrogen, both inorganic and organic (McKane et al., 2002; Weigelt et al., 2005; Harrison et al., 2007). Therefore, climate-driven changes in vegetation composition will alter nutrient competition between plant species, and between plants and soil microbes, with potential consequences for ecosystem nutrient cycling and soil carbon exchange. Our understanding of the importance for carbon exchange of such feedbacks between climate change, vegetation and soil microbial functioning is poor and hence represents an important research challenge.
Multiple global change drivers and soil microbes
Most studies to date on the effects of climate change on biological systems and soil microbes have examined single factors, such as elevated atmospheric CO2 concentration, warming, or drought. However, there is considerable potential for interactions between these factors to have additive or antagonistic effects on soil microorganisms and their activities related to greenhouse gas production (Shaw et al., 2002; Mikkelsen et al., 2008). Very little is known about the effects of multiple and interacting climate drivers on soil microbes and their contribution to climate change, and, being so complex, they are likely to be very difficult, if not impossible to predict. However, while some studies show unpredictable responses of soil microbial communities and their activities to combined effects of elevated temperature and atmospheric CO2 (for example, Kandeler et al., 1998), others point to strong additive effects with significant potential to feedback on carbon exchange. For example, the combined and positive effect of elevated temperature and atmospheric CO2 on microbial decomposition of peat was found to be greater than when these factors operated alone (Fenner et al., 2007a, 2007b), creating an even stronger positive feedback on carbon loss from soil as DOC and respiration (Freeman et al., 2004b). Added to this complexity is the knowledge that other organisms and trophic groups that influence soil microbes directly, such as microbial-feeding fauna (Cole et al., 2000; Johnson et al., 2005), or indirectly through altering vegetation diversity and productivity (for example, herbivores, plant pathogens and parasites) (Van der Putten et al., 2001; Wardle et al., 2004) or patterns of root exudation (Denton et al., 1999; Ayres et al., 2007), will also respond to multiple climate change factors (Wardle, 2002; Bardgett, 2005). This complexity further hampers our ability to predict effects of multiple climate change drivers on soil microbial communities and carbon exchange feedbacks.
The picture gets even more complex when we consider other global change phenomena, such as N deposition, invasion of new species and land use change, which all have the potential to individually influence soil microbes through a variety of direct and indirect pathways, but also interact with climate change. For example, N enrichment has direct and differential impacts on extracellular enzymes involved in decomposition processes (Carreiro et al., 2000; Frey et al., 2004), and on the abundance and diversity of different components of the soil microbial community, including bacteria, saprophytic fungi (Donnison et al., 2000; Bardgett et al., 2006) and mycorrhizal fungi (Egerton-Warburton and Allen, 2000; Frey et al., 2004), which are also affected directly and indirectly by climate change. Also, N deposition can indirectly influence soil microbes and decomposition processes through altering vegetation composition and productivity (for example, Stevens et al., 2004; van der Heijden et al., 2008) and by alleviating progressive N limitation of plant growth, which typically occurs under elevated atmospheric CO2 (Finzi et al., 2002). Remarkably little is known about effects of combined global changes on soil microbial communities, but they clearly have the potential to amplify, suppress or perhaps even neutralize climate change driven effects on soil microbes and their feedback to carbon exchange. We argue that future studies need to take a multifactor experimental approach to understand soil microbial responses to global changes and their consequences for carbon cycle feedbacks.
Conclusions and future challenges
An understanding of soil microbial ecology is central to our ability to assess terrestrial carbon cycle–climate feedbacks. However, the complexity of the soil microbial community and its many roles, coupled with the myriad of ways that climate and other global changes can affect soil microbes, hampers our ability to draw firm conclusions on this topic. Despite this uncertainty, we argue that progress can be made in understanding the potential negative and positive contributions of soil microbes to global warming through consideration of both direct and indirect impacts of climate change on microorganisms and the capacity for such effects to amplify or dampen carbon cycle feedbacks. This is a major challenge, but we believe that progress can be made through the use of long-term multifactor field experiments in relevant biomes, which incorporate consideration of direct and indirect impacts of climate change on soil microbes and their contribution to land–atmosphere carbon exchange, measured at the whole ecosystem scale. Such studies require a collaborative approach to link microbial ecology to whole ecosystem scale flux measures and modelling of carbon cycle feedbacks.
We have only scratched the surface of the contribution of soil microbes to climate change, and, as highlighted above, there are many uncertainties and challenges. In addition to what is mentioned above, we identify three major challenges. First, soil microbial communities are extremely diverse, and one of the greatest challenges concerns understanding how microbial diversity responds to climate change and the functional consequences of this for ecosystem carbon exchange, including the uptake, stabilization and release of carbon from soil as greenhouse gas. The major hurdle here is that many microbes are uncultivable, and the function of these noncultivable microbes is poorly understood because it is difficult to test how they respond to, or modify, their environment (van der Heijden et al., 2008). However, new molecular and stable isotope probing (SIP) tools are being developed that enable linking of changes in microbial diversity to ecosystem function, by focussing on functional genes that are important for biogeochemical processes and through directly labelling DNA, RNA and phospholipid fatty acids (PLFA) of organisms participating in particular pathways (Zak et al., 2006; Drigo et al., 2007; Neufeld et al., 2007). These tools have changed the way microbial ecologists explore the ecophysiology of microbial populations in the natural environment, because they enable study of the metabolic capabilities of uncultivable microorganisms, thus providing insights into the underlying processes regulating carbon flow in through different components of the soil microflora. For example, RNA-SIP approaches have been used to demonstrate differential carbon consumption among root-inhabiting microbes, including arbuscular mycorrhizal fungi (Vandenkoornhuyse et al., 2007) and incorporation of plant-derived 13C by Archaea (Yahai and Conrad, 2005), pointing to the importance of these microorganisms in regulating soil carbon flow. Biomarkers have also been used to indicate that responses of soil-borne communities to elevated CO2 are different for bacteria, fungi and nematodes and vary with plant type and soil nutrient availability (Drigo et al., 2007). Second, as already discussed, the diversity of carbon substrates in soil is considerable, and a major challenge is to understand the complexity of this carbon and how climate change and its interaction with other environmental factors affects its availability to enzymes that catalyze its degradation. Finally, as discussed in this paper, soil microbes and their activities are inextricably linked to aboveground communities, including plants, herbivores, pathogens and parasites. Understanding the effects of climate change on carbon dynamics therefore requires explicit consideration of the feedbacks that occur between aboveground and belowground communities and their response to climate change.
References
Aerts R, Chapin FS . (2000). The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30: 1–67.
Albers D, Migge S, Schaefer M, Scheu S . (2004). Decomposition of beech leaves and spruce needles in pure and mixed stands of beech and spruce. Soil Biol Biochem 36: 155–164.
Anisimov OA, Nelson FE, Pavlov AV . (1999). Predictive scenarios of permafrost development under conditions of global climate change in the XXI century. Earth Cryology 3: 15–25.
Ayres E, Ostle NJ, Cook R, Bardgett RD . (2007). The influence of below-ground herbivory and defoliation of a legume on nitrogen transfer to neighbouring plants. Funct Ecol 21: 256–263.
Bahn M, Kutsch WL, Heinemeyer A . (2008). Synthesis: emerging issues and challenges for an integrated understanding of soil carbon fluxes. In: Kutsch WL, Bahn M, Heinemeyer A (eds). Soil Carbon Fluxes. An Integrated Methodology. Cambridge University Press:Cambridge, (in press).
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK . (2005). A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20: 634–641.
Bardgett RD, Kandeler E, Tscherko D, Hobbs PJ, Jones TH, Thompson LJ et al. (1999). Below-ground microbial community development in a high temperature world. Oikos 85: 193–203.
Bardgett RD, Smith RS, Shiel RS, Peacock S, Simkin JM, Quirk H et al. (2006). Parasitic plants indirectly regulate belowground properties in grassland ecosystems. Nature 439: 969–972.
Bardgett RD . (2005). The Biology of Soil. A Community and Ecosystem Approach. Oxford University Press: Oxford.
Beedlow PA, Tingey DT, Phillips DL, Hogsett W, Olszyk DM . (2004). Rising atmospheric CO2 and carbon sequestration in forests. Front Ecol Env 2: 315–322.
Benoit RE, Starkey RL . (1968). Enzyme inactivation as a factor in the inhibition of decomposition of organic matter by tannins. Soil Science 105: 203–208.
Birch H . (1958). The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10: 9–31.
Carreiro MM, Sinsabaugh RL, Repert DA, Pankhurst DF . (2000). Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81: 2359–2365.
Christensen S, Tiedje JM . (1990). Brief and vigorous N2O production by soil at spring thaw. J Soil Sci 41: 1–4.
Cole L, Bardgett RD, Ineson P . (2000). Enchytraeid worms (Oligochaeta) enhance carbon mineralization in organic upland soils. Eur J Soil Sci 51: 185–192.
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF . (2008). Sensitivity of organic matter decomposition to warming varies with its quality. Global Change Biol 14: 868–877.
Conen F, Leifeld J, Seth B, Alewell C . (2006). Warming mobilises young and old soil carbon equally. Biogeosciences 3: 515–519.
Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, Alatalo J et al. (2007). Global negative feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10: 619–627.
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ . (2000). Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408: 184–187.
Curtis PS, Wang XZ . (1998). A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecolegia 113: 299–313.
Danby RK, Hik DS . (2007). Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. J Ecol 95: 352–363.
Davidson EA, Janssens IA . (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173.
De Deyn GB, Cornelissen HC, Bardgett RD . (2008). Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11: 516–531.
Denton CS, Bardgett RD, Cook R, Hobbs PJ . (1999). Low amounts of root herbivory positively influences the rhizosphere microbial community of a temperate grassland soil. Soil Biol Biochem 31: 155–165.
Díaz S, Grime JP, Harris J, McPherson E . (1993). Evidence of a feedback mechanism limiting plant-response to elevated carbon-dioxide. Nature 364: 616–617.
Dijkstra FA, Cheng WX . (2007). Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol Lett 10: 1046–1053.
Donnison LM, Griffith GS, Hedger J, Hobbs PJ, Bardgett RD . (2000). Management influences on soil microbial communities and their function in botanically diverse haymeadows of northern England and Wales. Soil Biol Biochem 32: 253–263.
Dorrepaal E, Cornelissen JHC, Aerts R, Wallen B, van Logtestijn RSP . (2005). Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along a latitudinal gradient? J Ecol 93: 817–828.
Dowrick DJ, Hughes S, Freeman C, Lock MA, Reynolds BR, Hudson JA . (1999). Nitrous oxide emissions from a gully mire in mid-Wales UK, under simulated summer drought. Biogeochem 44: 151–162.
Drigo B, Kowalchuk GA, Yergeau E, Bezemer TM, Boschker HTS, Van Veen JA . (2007). Impact of elevated carbon dioxide on the rhizosphere communities of Carex arenaria and Festuca rubra. Global Change Biol 13: 2396–2410.
Egerton-Warburton LM, Allen EB . (2000). Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10: 484–496.
Engelbrecht BM, Comita LS, Condit R, Kursar TA, Tyree MT, Turner BL et al. (2007). Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80–82.
Epstein HE, J Beringer WA, Gould AH, Lloyd AH, Thompson CD, Chapin FS et al. (2004). The nature of spatial transitions in the Arctic. J Biogeog 31: 1917–1933.
Fang CM, Smith P, Moncrieff JB, Smith JU . (2005). Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433: 57–59.
Fenner N, Freeman C, Lock MA, Harmens H, Sparks T . (2007b). Interactions between elevated CO2 and warming could amplify DOC exports from peatland catchments. Env Sci Technol 41: 3146–3152.
Fenner N, Ostle NJ, McNamara N, Sparks T, Freeman C . (2007a). Elevated CO2 effects on peatland plant community carbon dynamics and DOC production. Ecosystems 10: 635–647.
Fetzer S, Bak F, Conrad R . (1993). Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation. FEMS Microbiol Ecol 12: 107–115.
Fierer N, Craine JM, McLauchlan K, Schimel JP . (2005). Litter quality and the temperature sensitivity of decomposition. Ecology 86: 320–326.
Fierer N, Schimel J . (2002). Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34: 777–787.
Fierer N, Schimel J . (2003). A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67: 798–805.
Finzi AC, DeLucia EH, Hamilton JG, Richter DD, Schelsinger WH . (2002). The nitrogen budget of a pine forest under free-air CO2 enrichment. Oecologia 132: 567–578.
Fontaine S, Barot S . (2005). Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8: 1075–1087.
Freeman C, Fenner N, Ostle NJ, Kang H, Dowrick DJ, Reynolds B et al. (2004b). Dissolved organic carbon export from peatlands under elevated carbon dioxide levels. Nature 430: 195–198.
Freeman C, Nevison GB, Kang H, Hughes S, Reynolds B, Hudson JA . (2002). Contrasted effects of simulated drought on the production and oxidation of methane in a mid-Wales wetland. Soil Biol Biochem 34: 61–67.
Freeman C, Ostle NJ, Fenner N, Kang H . (2004a). A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol Biochem 36: 1663–1667.
Freeman C, Ostle NJ, Kang H . (2001). An enzymic ‘latch’ on a global carbon store. Nature 409: 149.
Frey SD, Knorr M, Parrent JL, Simpson RT . (2004). Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecol Manag 196: 159–171.
Friedlingstein P, Cox P, Betts R, Bopp L, Von Bloh W, Brovkin V et al. (2006). Climate-carbon cycle feedback analysis: results from the (CMIP)-M-4 model intercomparison. J Climate 19: 3337–3353.
Gamper H, Hartwig UA, Leuchtmann A . (2005). Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 yr of selection under elevated atmospheric CO2 partial pressure. New Phytol 167: 531–542.
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL . (2001). Colder soils in a warmer world: a snow manipulation study in northern hardwood forest. Biogeochem 56: 135–150.
Hanley ME, Trofmov S, Taylor G . (2004). Species-level effects more important than functional group-level responses to elevated CO2: evidence from simulated turves. Funct Ecol 18: 304–313.
Harrison KA, Bol R, Bardgett RD . (2007). Preferences for uptake of different nitrogen forms by co-existing plant species and soil microbes in temperate grasslands. Ecology 88: 989–999.
Heath J, Ayres E, Possell M, Bardgett RD, Black HIJ, Grant H et al. (2005). Rising atmospheric CO2 reduces soil carbon sequestration. Science 309: 1711–1713.
Heimann M, Reichstein M . (2008). Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451: 289–292.
Henry H, Juarez JD, Field CB, Vitousek PM . (2005). Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a Californian annual grassland. Global Change Biol 11: 1808–1815.
Högberg P, Read DJ . (2006). Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol 21: 548–554.
Houghton JT, Meira Filho LG, Callender BA, Harris N, Kattenberg A, Maskell K . (1996). Climate Change 1995. The Science of Climate Change. Intergovernmental Panel on Climate Change. Cambridge University Press: Cambridge, UK.
Hu S, Chapin FS, Firestone MK, Field CB, Chiariello NR . (2001). Nitrogen limitation of microbial decomposition in a grassland under elevated CO2 . Nature 409: 188–191.
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED . (1996). A global analysis of root distributions for terrestrial biomes. Oecologia 108: 389–411.
Jenkinson DS, Adams DE, Wild A . (1991). Model estimates of CO2 emissions from soil in response to global warming. Nature 351: 304–306.
Johnson D, Kresk M, Stott AW, Cole L, Bardgett RD, Read DJ et al. (2005). Soil invertebrates disrupt carbon flow through fungal networks. Science 309: 1047.
Kandeler E, Tscherko D, Bardgett RD, Hobbs PJ, Kampichler C, Jones TH . (1998). The response of soil microorganisms and roots to elevated CO2 and temperature in a terrestrial model ecosystem. Plant Soil 202: 251–262.
Keel SG, Siegwolf RTW, Körner C . (2006). Canopy CO2 enrichment permits tracing the fate of recently assimilated carbon in a mature deciduous forest. New Phytologist 172: 319–329.
King GM . (1992). Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv Microb Ecol 12: 431–468.
Kirschbaum MUF . (2004). Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Global Change Biol 10: 1870–1877.
Kirschbaum MUF . (2006). The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem 38: 2510–2518.
Klironomos JN, Rillig MC, Allen MF, Zak DR, Kubiske M, Pregitzer KS . (1997). Soil fungal-arthropod responses to Populus tremuloides grown under enriched atmospheric CO2 under field conditions. Global Change Biol 3: 473–478.
Kluber HD, Conrad R . (1998). Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol Ecol 25: 301–318.
Knorr W, Prentice IC, House JI, Holland EA . (2005). Long-term sensitivity of soil carbon turnover to warming. Nature 433: 298–301.
Krivtsov V, Bezginova T, Salmond R, Liddell K, Garside A, Thompson J et al. (2006). Ecological interactions between fungi, other biota and forest litter composition in a unique Scottish woodland. Forestry 79: 201–216.
Kuzyakov Y . (2006). Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38: 425–448.
Luo Y, Wan S, Hui D . (2001). Acclimization of soil respiration to warming in tall grass prairie. Nature 413: 622–625.
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S et al. (2007). The human footprint in the carbon cycle of temperate and boreal forests. Nature 447: 848–850.
Matzner E, Borken W . (2008). Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review Eur J Soil Sci 59: 274–284.
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B et al. (2002). Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415: 68–71.
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP et al. (2002). Soil warming and carbon-cycle feedbacks to the climate system. Science 298: 2173–2175.
Mikkelsen C, Beier S, Jonasson M, Holmstrup IK, Schmidt P, Ambus K et al. (2008). Experimental design of multifactor climate change experiments with elevated CO2, warming and drought: the CLIMAITE project. Funct Ecol 22: 185–195.
Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW et al. (2006). Winter soil respiration controlled by climate and microbial community composition. Nature 439: 711–714.
Monteith DT, Stoddard JL, Evans CD, de Wit HA, Forsius M, Hogasen T et al. (2007). Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537–539.
Nardo CD, Cinquegrana A, Papa S, Fuggi A, Fioretto A . (2004). Laccase and peroxidase isoenzymes during leaf litter decomposition of Quercus ilex in a Mediterranean ecosystem. Soil Biol Biochem 36: 1539–1544.
Neufeld JD, Wagner M, Murrell JC . (2007). Who eats what, where and when? Isotope-labelling experiments are coming of age. The ISME Journal 1: 103–110.
Prentice IC, Cramer W, Harisson SP, Leemans R, Monserud RA, Solomon AM . (1992). A global biome model based on plant physiology and dominance, soil properties and climate. J Biogeogr 19: 117–134.
Read DJ, Leake JR, Perez-Moreno J . (2004). Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 82: 1243–1263.
Rey A, Jarvis P . (2006). Modelling the effect of temperature on carbon mineralization rates across a network of European forest sites (FORCAST). Global Change Biol 12: 1894–1908.
Rillig MC, Mummey DL . (2006). Mycorrhizas and soil structure. New Phytol 171: 41–53.
Ross DJ, Newton PCD, Tate KR . (2004). Elevated CO2 effects on herbage and soil carbon and nitrogen pools and mineralization in a species–rich, grazed pasture on a seasonally dry sand. Plant Soil 260: 183–196.
Roulet NT, Moore TR . (1995). The effect of forestry drainage practices on the emissions of methane from northern peatlands. Can J Forest Res 25: 491–499.
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS et al. (2005). Determinants of woody cover in African savannas. Nature 438: 846–849.
Schimel DS, Braswell BH, Holland EA, McKeown R, Ojima DS, Painter TH et al. (1994). Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils, Global Biogeochem. Cycles 8: 279–293.
Schimel JP, Balser TC, Wallenstein M . (2007). Microbial stress-response physiology and its implications for ecosystem function. Ecology 88: 1386–1394.
Schimel JP, Mikan C . (2005). Changing microbial substrate use in Arctic tundra soils through a freeze-thaw cycle. Soil Biol Biochem 37: 1411–1418.
Sharma S, Szele Z, Schilling R, Munch JC, Schloter M . (2006). Influence of freeze-thaw on the structure and function of microbial communities in soil. Appl Environ Microbiol 72: 48–54.
Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB . (2002). Grassland responses to global environmental changes suppressed by elevated CO2 . Science 298: 1987–1990.
Sitch S, Cox PM, Collins WJ, Huntingford C . (2007). Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448: 791–794.
Six J, Frey SD, Thiet RK, Batten KM . (2006). Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70: 555–569.
Smith P, Martino D, Cai Z, Gwary D, Janzen HH, Kumar P et al. (2008). Greenhouse gas mitigation in agriculture. Phil Trans Royal Soc B 363: 789–813.
Staddon PL, Jakonsen I, Blum H . (2004). Nitrogen input mediates the effects of free-air CO2 enrichment on mycorrhizal fungal abundance. Global Change Biol 10: 1687–1688.
Stevens CJ, Dise NB, Mountford JO, Gowing DJ . (2004). Impact of nitrogen deposition on the species richness of grasslands. Science 303: 1876–1879.
Ström L, Mastepanov M, Christensen TR . (2005). Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochem 75: 65–82.
Trumbore S . (2006). Carbon respired by terrestrial ecosystems—recent progress and chellanges. Global Change Biol 12: 141–153.
Van der Heijden MGA, Bardgett RD, van Straalen NM . (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11: 296–310.
Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL . (2001). Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16: 547–554.
Vandenkoornhuyse P, Mahé S, Ineson P, Staddon P, Ostle P, Cliquet JB et al. (2007). Active root-inhabiting microbes identified by rapid incorporation of plant-derived carbon into RNA. Proc Natl Acad Sci USA 104: 16970–16975.
Ward SE, Bardgett RD, McNamara NP, Adamson JK, Ostle NJ . (2007). Long-term consequences of grazing and burning on northern peatland carbon dynamics. Ecosystems 10: 1069–1083.
Wardle DA . (2002). Communities and Ecosystems: Linking the Aboveground and Belowground Components. Princeton University Press: Princeton, New Jersey.
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH . (2004). Ecological linkages between aboveground and belowground biota. Science 304: 1634–1637.
Weigelt A, Bol R, Bardgett RD . (2005). Preferential uptake of soil N forms by grassland plant species. Oecologia 142: 627–635.
Woodward FI, Lomas MR, Kelly CK . (2004). Global climate and the distribution of plant biomes. Proc R Soc Lond Ser B Biol Sci 359: 1465–1476.
Yahai L, Conrad R . (2005). In Situ Stable Isotope Probing of Methanogenic Archaea in the Rice Rhizosphere. Science 309: 1088–1090.
Zak DR, Blackwood CB, Waldrop MP . (2006). A molecular dawn for biogeochemistry. Trends Ecol Evol 21: 288–295.
Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL . (1993). Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151: 105–117.
Zibilske LM, Bradford JM . (2007). Oxygen effects on carbon, polyphenols, and nitrogen mineralization potential in soil. Soil Sci Soc Am J 71: 133–139.
Acknowledgements
We are extremely grateful to George Kowalchuk for inviting us to write this article and, along with two anonymous referees, for providing helpful comments on the paper. Many of the ideas in this paper emerged from discussion with colleagues involved in the QUERCC (Quantifying ecosystem roles in the carbon cycle) project which is supported by NERC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardgett, R., Freeman, C. & Ostle, N. Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2, 805–814 (2008). https://doi.org/10.1038/ismej.2008.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2008.58
Keywords
This article is cited by
-
An insight on the contributions of microbial communities and process parameters in enhancing biogas production
Biomass Conversion and Biorefinery (2024)
-
Litter Decomposition Rates in a Post-mined Peatland: Determining Factors Studied in Litterbag Experiments
Environmental Processes (2024)
-
Bacterial communities in cropland soils: Taxonomy and functions
Plant and Soil (2024)
-
A framework for the targeted recruitment of crop-beneficial soil taxa based on network analysis of metagenomics data
Microbiome (2023)
-
The core mangrove microbiome reveals shared taxa potentially involved in nutrient cycling and promoting host survival
Environmental Microbiome (2023)