Abstract
In silico, physiological and in planta analyses were used to characterize pBTAi1, a 229-kb accessory plasmid from Bradyrhizobium sp. strain BTAi1, and assess its potential ecological function under free-living and symbiotic growth conditions. Sequence analysis revealed the presence of an uptake hydrogenase system, a repABC family plasmid replication module and open reading frames encoding type IV secretion system, TraI and TraR autoinducer proteins and several copper resistance-related proteins. Bradyrhizobium sp. BTAi1 was capable of growing in 200 mg l−1 CuCl2. In contrast, the closely related, plasmid-free Bradyrhizobium sp. strain ORS278 could not grow at copper concentrations exceeding 100 mg l−1. The plasmid-localized hydrogenase genes were phylogenetically distinct from those typically found in other rhizobial species, and were most related to hup genes from Thiobacillus denitrificans. The induction of the plasmid-borne hydrogenase genes during symbiosis was significantly lower than the two chromosomal-borne hydrogenase clusters. CHEF-pulsed-field gel electrophoresis was used for a comprehensive analysis of the diversity, abundance and genetic composition of accessory plasmids in other Bradyrhizobium strains. Plasmids were detected in 11 of 46 (23.9%) geographically diverse Bradyrhizobium japonicum and Bradyrhizobium elkanii strains, isolated from the United States, China and Thailand. Plasmid size was heterogeneous, ranging from 75 to 330 kb, with only two strains (DASA01244 and DASA01265) harboring plasmids with identical (240 kb) size. None of the plasmids harbored nodulation or hydrogenase genes. Taken together, our results indicate that while plasmids having ecologically significant functions may be detected in Bradyrhizobium sp. strains, they lack genes necessary for symbioses with legumes.
Similar content being viewed by others
Introduction
The primary source of biologically-fixed nitrogen (N2) in agricultural systems is produced via the symbiotic interaction between legumes and the root- and shoot-nodulating soil bacteria, collectively referred to as the rhizobia. The rhizobia can be divided into three broad groups based on their speed of growth on laboratory media: the fast growers, which consist of strains in the genera Rhizobium, Sinorhizobium and Azorhizobium; the intermediate growth rate strains in the genus Mesorhizobium and the slow growers, which comprise strains in the phylogenetically distinct genus Bradyrhizobium (Sawada et al., 2003).
Fast- and slow-growing rhizobia are also unique with respect to the location of genes involved in nodulation and symbiotic nitrogen fixation. Generally speaking, the structural genes for the nitrogenase enzyme (nifHDK) and genes essential for the production of lipochitooligosaccharide nodulation factors (nodABC) in the fast-growing species are located on large, high-molecular weight, plasmids (pSym; Merrick and Edwards, 1995). Plasmids can vary significantly between and within species of rhizobia, ranging from 180 kb in Rhizobium leguminosarum bv. trifolii, to 1.35 Mb in Sinorhizobium meliloti (Mercado-Blanco and Toro, 1996; Barnett et al., 2001). In contrast, genes encoding nod factors and nitrogenase in Bradyrhizobium species and most mesorhizobia are located on the chromosome in regions termed ‘symbiosis islands’ (Sawada et al., 2003). In the soybean symbiont Bradyrhizobium japonicum, symbiosis-related and hydrogenase genes are localized to on a 611-kb chromosomal-borne symbiosis island that displays a distinct distribution of insertion sequence elements relative to the rest of the chromosome (Gottfert et al., 2001; Kaneko et al., 2002). Interestingly, while Mesorhizobium loti has a chromosomal-borne symbiosis island, the moderately slow-growing Mesorhizobium amorphae was found to carry a 930-kb pSym (Wang et al., 1999).
Several Rhizobium and Bradyrhizobium species contain uptake hydrogenases, which reduce energy loss associated with symbiotic nitrogen fixation (Albrecht et al., 1979; Evans et al., 1987). Generally, the hydrogen uptake (hup) genes are arranged in at least three operons with conserved gene composition and organization (Baginsky et al., 2002). The hupS and hupL genes encode for hydrogenase structural subunits, whereas the remaining hup and hyp gene products encode for maturation proteins, which are responsible for the recruitment and incorporation of nickel and other metals into the hydrogenase active site (Casalot and Rousset, 2001). While the R. leguminosarum and B. japonicum hydrogenase systems are highly homologous, they differ in gene regulation and in the presence of specific genes. In Rhizobium tropici and R. leguminosarum the hup genes are encoded on the symbiotic plasmid, whereas in B. japonicum, Bradyrhizobium sp. (Lupinus) and Bradyrhizobium sp. (Vigna), the hup genes have been presumed to be on the chromosome, since no plasmids could be detected in the strains examined by using standard plasmid isolation procedure (Baginsky et al., 2002; Brito et al., 2005).
In addition to symbiotic plasmids, several rhizobial strains also contain one or more smaller, accessory plasmids, also termed non-symbiotic plasmids (Mercado-Blanco and Toro, 1996; Gonzalez et al., 2006; Stiens et al., 2006; Young et al., 2006). Some of these plasmids have been shown to transfer between populations of indigenous rhizobia (Mercado-Blanco and Toro, 1996). The acquisition of some accessory plasmids has been correlated with enhanced symbiotic performance (Martínez et al., 1987), while others have been shown to enhance growth under specific environmental conditions (Mercado-Blanco and Toro, 1996). Both symbiotic and accessory plasmids require replication control mechanisms that ensure proper segregation and efficient maintenance of the low-copy number DNA replicons in their host bacterium (Ramirez-Romero et al., 2000).
In contrast to the significant number of reports of plasmids in the fast-growing rhizobia, there have been relatively few studies concerning the presence of plasmids in Bradyrhizobium strains, perhaps due to methodological limitations. While a few studies have reported the presence of two to four plasmids in B. japonicum strains, ranging in size from 74 to 294 kb (Gross et al., 1979; Masterson et al., 1982), the taxonomic status of some of the strains was not verified using molecular, phylogenetic tools (Gross et al., 1979; Masterson et al., 1982). Although Masterson et al. (1982) showed that nod genes were not found on any plasmids they detected, no information was presented regarding the genetic composition of these plasmids, and thus the physiological role of plasmids in bradyrhizobia could not be inferred. In addition, due to changes in the taxonomy of soybean-nodulating bacteria, some strains have since been reclassified as belonging to Sinorhizobium fredii, a fast-growing species. To date, the functional role of plasmids in the bradyrhizobia has not been explored, and until now (Giraud et al., 2007) there have been no reports on the complete sequence and analysis of plasmids from any Bradyrhizobium strain.
The Bradyrhizobium strain BTAi1 belongs to a novel group of phototrophic bradyrhizobia that form a symbiotic association with several species in the legume genus Aeschynomene. Members of this bacterial group although taxonomically related to other bradyrhizobia, are unique in their ability to couple photosynthesis to nitrogen fixation (Giraud and Fleischman, 2004). Recently, it was reported that Bradyrhizobium sp. strain BTAi1, and other bradyrhizobia capable of forming nodules on Aeschynomene indica, are taxonomically related to Blastobacter denitrificans, and it has been proposed that the later bacterium be transferred to the genus Bradyrhizobium (van Berkum et al., 2006). To date, however, Bradyrhizobium strains capable of nodulating Aeschynomene have not been given specific species status.
A comprehensive description of the structural and functional genomics of strain BTAil was recently described, revealing the presence of a 229-kb accessory plasmid (Giraud et al., 2007). The presence of plasmids in Bradyrhizobium sp. strains capable of nodulating Aeschynomene appears not to be a common feature of this group of bacteria, as no plasmid was identified in the closely related strain ORS278 (Giraud et al., 2007). In this study we used in silico, physiological and in planta analyses to understand the mode of regulation, phylogenetic affiliation and potential environmental role of the accessory plasmid pBTAi1 from Bradyrhizobium sp. strain BTAi1. This is, to our knowledge, the first comprehensive sequence analysis of a plasmid from a member of the genus Bradyrhizobium. We also report here on the presence of other accessory plasmids in genetically diverse B. japonicum and B. elkanii strains isolated from three geographically distinct regions of the world. These plasmids, however, do not contain symbiosis-related nodulation and uptake hydrogenase genes.
Materials and methods
Bacterial strains, media and growth conditions
The strains used in this study are listed in Table 1. All B. japonicum strains were re-isolated from soybean nodules by plant infection assay (Somasagaran and Hoben, 1994), and streaked several times for single colonies onto yeast extract-mannitol agar medium containing either bromthymol blue or congo red as indicators (Somasagaran and Hoben, 1994). All strains were grown at 30 °C.
Sequence analysis of nearly full-length 16S rDNA was performed to determine the species status of the Thai Bradyrhizobium strains used in these studies. Polymerase chain reaction was performed using 16S rRNA gene primers 27F, 5′-AGAGTTTGATCMTGGCTCAG-3′, and 1525R, 5′-AAGGA GGTGWTCCARCC-3′ (Lane, 1991). The following reaction conditions were used: 95 °C for 5 min, followed by 30 cycles consisting of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 90 s. After final extension at 72 °C for 5 min, PCR reaction mixtures were stored at 4 °C. Electrophoresis was performed at 100 V for 2.5 h. DNA fragments about 1.5 kb in size were recovered from gels, purified using a QIAquick Kit (Qiagen, Valencia, CA, USA) and directly used as templates for sequencing using primers 27F and 1525R. Sequences of 16S rDNA were aligned and identified by using the BLASTN algorithm (http://www.ncbi.nlm.nih.gov/BLAST/).
Preparation of high-molecular-weight total of genomic DNA
High-molecular-weight total genomic DNA from Bradyrhizobium strains was isolated using an embedded agarose method (Birren and Lai, 1993). Strains were cultivated in Arabinose-Gluconate (AG) medium (Sadowsky et al., 1987) to OD600 of about 0.5. Cells were harvested by centrifugation at 8000 g at 4 °C, washed twice with TE buffer (20 mM Tris and 50 mM ethylenediamine tetraacetic acid, pH 8.0) and resuspended in TEN buffer (10 mM Tris, 20 mM NaCl and 50 mM ethylenediamine tetraacetic acid, pH 7.2) to an OD600 of 0.6–0.7. Cell suspensions were combined with an equal volume of 2% (w/v) low-melting-point SeaPlaque GTG Agarose (BMA, Rockland, ME, USA) prepared in TE buffer containing 1% sodium dodecyl sulfate, and plugs were prepared using a plug mold (BioRad, Hercules, CA, USA). Plugs were treated with Lysozyme buffer, Proteinase K buffer, and wash buffer containing 1 mM phenylmethyl sulforyl fluoride (Sigma-Aldrich, St Louis, MO, USA) at room temperature for 30 min as previously described (Birren and Lai, 1993). The agarose plugs were stored in TE buffer at 4 °C until use.
Plasmid profiling
Plasmid profiles were obtained using a combination of S1-nuclease digestion and pulsed-field gel electrophoresis (PFGE)-contour clamped homogenous electric field (CHEF) as previously described (Sajjaphan et al., 2006). DNA plugs were incubated at 37 °C for 10 min with 5 U of Aspergillus oryzae S1 nuclease (Invitrogen, Carlsbad, CA, USA) as described (Barton et al., 1995). PFGE-CHEF was performed using a CHEF-DRII apparatus (BioRad) and gels were run for 22 h at 14 °C in 0.5 × TBE buffer at 6 V cm−1 (45 mM Tris base, 45 mM boric acid and 1 mM ethylenediamine tetraacetic acid, pH 8.0), with switch times ramped from 1.0 to 40.0 s. A λ-PFG ladder (New England Biolabs, Ipswich, MA, USA) served as the molecular weight marker. Following electrophoresis, gels were stained for 20 min with ethidium bromide (1 μg ml−1) in TBE buffer, destained for 30 min in distilled water and plasmid bands were visualized using a UV transilluminator.
Southern hybridization analyses
Cosmid pR32 (Sadowsky et al., 1991), which was used as a nodulation (nod) gene probe, was isolated as described by Itoh et al. (1984). Plasmid pR32 was digested to completion with HindIII, releasing the nodulation genes nolA, nodD2, nodD1YABC, nodSUIJ and nodZ and 50 ηg of probe DNA was 32P-labeled using the Rediprime II random prime labeling system (Amersham Biosciences, Piscataway, NJ, USA). DNA from B. japonicum USDA110 served as the positive control for all hybridization reactions. Total genomic DNA was isolated as previously described (Sadowsky et al., 1987), digested with EcoRI and separated by agarose gel electrophoresis as described (Sadowsky et al., 1991). Intact (undigested) plasmid profiles obtained from PFGE-CHEF were transferred to Nytran immobilization membranes (Schleicher&Schuell, Keene, NH, USA) and hybridized to probes as previously described (Sadowsky et al., 1987).
Plasmid sequencing and phylogenetic analyses
Genomic DNA was isolated from Bradyrhizobium sp. BTAi1 as previously described (Sadowsky et al., 1987), and sequencing was performed at the DOE Joint Genome Institute (Giraud et al., 2007). Genes on pBTAi1 were annotated using the Mage Microbial Genome Annotation System (Vallenet et al., 2006) as previously described (Giraud et al., 2007). Plasmid sequence data have been deposited in GenBank under accession number NC_009475.
Amino-acid (AA) sequences were aligned by using ClustalW (http://align.genome.jp/), and bootstrap analyses and phylogenetic trees were generated using the maximum likelihood method from the Phylip phylogenetic package (Felsenstein, 1989).
Plant assays
A. indica seeds were surface sterilized in concentrated sulfuric acid for 1 h, thoroughly rinsed and soaked overnight in sterile water and seeds were transferred to water agar (8 g l−1) plates for 20 h at 35 °C for germination. Plants were grown in Leonard jar assemblies, two plants per jar, as previously described (Sadowsky et al., 1995) and watered with sterile N-free plant nutrient solution (Summerfield et al., 1977). Plants roots were inoculated with 1 ml of approximately 108 cells per ml of Bradyrhizobium sp. strain BTAi1. Seeds were covered with a 1-cm layer of sterilized paraffin-coated sand (Sadowsky et al., 1995) and plants were grown 30–45 days in a growth chamber, with 16 h of light and day and night temperatures of 28 and 23 °C, respectively. After three weeks of growth, plant stems were inoculated by carefully applying ∼108 cells in sterile water to the stems using a sterile cotton-swab applicator.
RNA extraction, reverse transcription and quantitative real-time PCR
RNA was extracted from AG-grown, log-phase Bradyrhizobium sp. BTAil cultures and A. indica root and stem nodules using the RNeasy purification kit (Qiagen) according to the manufacturer's instructions, with slight modification. RNA from nodule tissue (0.2 g) was extracted using glass beads and lysosyme solution (0.5 mg ml−1) and a Fast Prep bead beating system (Bio101, Vista, CA, USA).
Reverse transcription was performed using 0.5 μg of total RNA, 1 mM dNTP's, 1 × first-strand buffer, 15 mM DTT and 400 units Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Reactions were incubated for 3 h at 42 °C followed by an inactivation step of 65 °C for 20 min. RNA samples were treated with RNAse-free DNAse before reaction. Quantitative real-time PCR of the three BTAi1 hydrogenases, under both free-living and symbiotic in planta conditions, was performed using the primer sets listed in Table 2. Specificity of the primer sets was confirmed using negative controls consisting of DNA extracted from the phylogenetically similar Bradyrhizobium sp. strain ORS278 and from A. indica tissue. Primers targeting the hupL genes for each of the three Bradyrhizobium sp. BTAi1 hydrogenase clusters, and the housekeeping gene parA (BBta_0167), were designed using the online version of Primer 3 software (Rozen and Skaletsky, 2000). Each 25-μl reaction contained 0.5 μg of total cDNA, 8.75 μl dH2O, 12.5 μl Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), 0.25 μg μl−1 BSA and 0.2 μM each of forward and reverse gene-specific primers. The quantitative real-time PCR reactions were run on an Applied Biosystems 7500 real-time PCR system, using Sequence Detection System software (version 1.3; Foster City, CA, USA). The PCR program was as follows: 95 °C for 10 min followed by 45 cycles of 95 °C for 15 s and annealing and extension at 60 °C for 1 min. Expression values for three biological replicates for each treatment were normalized to the expression level of the BTAi1-encoded parA gene. The expression of parA in BTAi1 was previously shown to be stable under a variety of physiological conditions (Chang et al., unpublished observations; Cytryn et al., unpublished observations).
Hydrogenase activity
Hydrogenase activity was determined using a modification of a previously described method (Keyser et al., 1982). Fresh root or stem nodules (0.2–0.3 g) were homogenized in 5 ml HEPES buffer, pH 7.0, placed in 20 ml glass vials sealed with butyl rubber stoppers, and H2 was injected into the bottles to a final concentration of 5% (v/v). Bottles were incubated at 30 °C and hydrogen concentration in the headspace was periodically measured using a Hewlett-Packard 5890 Series II gas chromatograph containing a 6ft stainless steel, 1/8th inch inner diameter, 5A matrix 60/80 Molecular Sieve column (Supelco, Bellefonte, PA, USA) and a thermal conductivity detector. Hydrogenase activity was determined by measuring the rate at which the injected H2 was consumed in test vials in relation to control vials without added nodule tissue.
Results and discussion
Sequence analysis of accessory plasmid pBTAi1 from Bradyrhizobium sp. strain BTAi1
Complete sequencing of the Bradyrhizobium sp. strain BTAi1 genome revealed the presence of a circular plasmid, pBTAi1. The plasmid was 228 826 bp in length, had a mean G+C content of 60.71% (compared with 64.9% for the Bradyrhizobium sp. strain BTAi1 chromosome) and contained 257 predicted open reading frames (ORFs), of which 66.2% (174) are presently characterized as encoding hypothetical proteins. The plasmid contained 29 pseudogenes, and seven and 10 putative insertion elements and transposons, respectively. A full description of all ORFs present on pBTAi1 is shown in Supplementary Table 1.
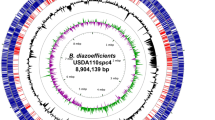
The circular map of plasmid pBTAi1 is shown in Figure 1. Six principal functional regions on pBTAi1 were defined based on AA identity and synteny to known genes in other microorganisms. The functional characterization of genes in these regions and the closest identity of corresponding ORFs in other microorganisms are summarized in Table 3. Region I (from nucleotides 192 to 3864) contained a repABC gene cluster and region II (nucleotides 34 100–38 204) encoded for a probable ABC-type AA transport system. Genes likely involved in heavy metal and copper resistance (nucleotides 84 302–89 201) were identified in regions III and IV, and region V (nucleotides 126 219–136 790) contained a gene cluster encoding an uptake hydrogenase. The region VI (nucleotides 180 987–192 253) contained genes encoding a type IV secretion system, the synthesis of autoinducer and the transcriptional activator proteins TraI and TraR, respectively. Details of principal genes in these regions are presented below.
Circular map of the Bradyrhizobium sp. strain plasmid pBTAi1. Functional regions are as described in Table 3. Regions: I, a RepABC replicon module; II, AA transport and metabolism proteins; III and IV, copper and metal resistance proteins; V, uptake hydrogenase and accessory proteins and VI, type IV secretion system and TraI/R quorum-sensing plasmid transfer system. The two circles outside and inside the red and blue arrows indicate the GC skew (positive and negative) of the genome regions transcribed in the clockwise and counterclockwise directions, respectively. Red and blue arrows represent predicted ORFs transcribed in the clockwise and counterclockwise directions, respectively. Pink and gray rectangles in the inner circle represent transposable elements and pseudogenes, respectively. AA, amino acid; ORF, open reading frame.
The repABC replicon of plasmid pBTAi1
The ORFs BBta_p0001 to BBta_p0003 encode for a RepABC-type replicon. These three ORFs form a monophyletic cluster with the repABC genes from a diversity of previously described α-proteobacterial plasmids. The repA and repB genes encode homologues of the ParAB family of replicon partitioning proteins (Bignell and Thomas, 2001), and repC is thought to encode for the replication initiation (replicase) protein (Ramirez-Romero et al., 2001). The pBTAi1 repA and repB gene products each shared 72% AA identity to repA and repB from the Agrobacterium rhizogens plasmid pRi1724 (accession number AP002086), and repC shared 89% AA identity to the repC gene of plasmid X14 (accession number YP_571716) from Nitrobacter hamburgensis. A large A+T-rich intergenic sequence, commonly found in other rhizobial plasmids, was found between repB and repC on the pBTAi1 plasmid (Ramirez-Romero et al., 2000). The repABC replicon family is required for ensuring proper segregation and efficient maintenance of low-copy DNA replicons (Ramirez-Romero et al., 2000), and has been extensively characterized and is genetically conserved in the α-proteobacteria. The repABC replicon has also been shown to control the maintenance of rhizobial symbiotic megaplasmids (Ramirez-Romero et al., 2000; Barnett et al., 2001), the Ti plasmids in Agrobacterium sp. strains (Li and Farrand, 2000) and the second chromosome in the animal pathogen Brucella (Paulsen et al., 2002). The repABC genes also appear to be widely distributed on accessory plasmids in several rhizobial species (Cevallos et al., 2002; Stiens et al., 2006; Watson and Heys, 2006).
Amino-acid sequence analysis revealed that two ORFs, BBta_p0014 and BBta_p0015, located downstream of the repABC replication module, were 42% and 35% identical to ParA-like ATPase and ParB-like partition protein from S. meliloti plasmid pMBA19a (accession number AY914874) and the Rhodobacter sphaeroides plasmid pRSPA04 (accession number NC_009432), respectively. The parA and parB, which are required for efficient plasmid and chromosome partitioning, are found on many low-copy-number plasmids and bacterial chromosomes (Bignell and Thomas, 2001). The existence of an alternative replication-control region in tandem to repABC has been previously described in other rhizobial plasmids, and is thought to broaden host range (Stiens et al., 2006; Watson and Heys, 2006).
Conjugation and gene transfer systems
Gene cluster VI (Table 3; Figure 1) contained 10 ORFs encoding VirB1 to VirB11 components of the TraG/VirD4 family type IV secretion system (T4SS). Although not detected in the original annotation, re-examination of the plasmid genome revealed the presence of a Vir B7 homologue (BBta_p0296) located between BBta_p0251 and BBta_p0252. Sequences from these ORFs formed a monophyletic clade with T4SS genes from plasmids in other members of the Rhizobiaceae, and were most similar to T4SS genes from S. meliloti 1021 pSymA (Table 3). Although presumably involved in inducing conjugative horizontal DNA transfer, very little data currently exist regarding the functional role of the plasmid-encoded T4SS in rhizobia. Stiens et al. (2006) however reported that the T4SS on pSmeSM11a from S. meliloti was not involved in mobilization of this plasmid, and S. meliloti strains containing virB operon deletions were not affected in nodulation or symbiotic nitrogen fixation (Barnett et al., 2001).
Plasmid pBTAi1 also contained traI and traR quorum sensing-related genes directly upstream from the T4SS genes. In some cases, repABC expression, and therefore plasmid copy number control is subject to TraR-mediated quorum sensing (He et al., 2003; Pappas and Winans, 2003). Control of the conjugal transfer of pBTAi1 may also be mediated by ORF BBta_p0279, which encodes a putative ArdC-like conjugal transfer antirestriction protein. This ORF forms a monophyletic clade with ArdC proteins from other α-proteobacterial plasmid, and is most closely related (74% AA identity) to ArdC from N. hamburgensis X14. The Ard proteins are thought to protect plasmid DNA from host restriction endonuclease activity during conjugal transfer (Belogurov et al., 2000).
Copper-resistance genes
The pBTAi1 regions III and IV (Table 3) contained several ORFs (BBta_p0056, BBta_p0061, BBta_p0114 to p0118) with high AA identity to copper-resistance proteins from other, closely related α-proteobacteria, including Rhodopseudomonas, and Nitrobacter species. These included components of a putative copABCD (pcoABCD) copper-resistance operon (BBta_p0114 to p0118) conferring structural resistance to high concentrations of copper (Rensing and Grass, 2003). At least seven additional ORFs that are putatively involved in copper resistance and transport were also detected throughout the pBTAi1 genome. These included additional copA- (BBta_p0190) and copB-like (BBta_p0189) ORFs located upstream of region II, and three putative copper-transporting P-type ATPases (BBta_p0056, BBta_p0121 and BBta_p0125). Copper resistance assays showed that Bradyrhizobium sp. BTAi1 has a relatively high level of copper resistance, with growth at concentrations of up to 200 mg l−1 CuCl2. In contrast, the closely related plasmid-free, phototrophic Bradyrhizobium sp. ORS278 could not grow at concentrations of CuCl2 exceeding 100 mg l−1, indicating a potential ecological role of the Bradyrhizobium sp. BTAi1 plasmid-borne copper-resistant genes. Although BTAi 1 was isolated from a submerged stem nodule on greenhouse-grown A. indica, the original inoculum originated from quartz sand rooting medium obtained from an open pit mine in West Virginia (Stowers and Eaglesham, 1983). Thus, enhanced heavy metal resistance, conferred by pBTAi1, might have provided an evolutionary advantage in this metal-rich environment. Plasmid-borne Pco/copABCD-type copper-resistance operons have been described in detail for Escherichia coli (Rensing and Grass, 2003) and Pseudomonas syringae pv. tomato (Mills et al., 1994). Moreover, genomic analysis has revealed a large number of similar genes on bacterial plasmids and chromosomes for a variety of other microorganisms (Mergeay et al., 1995; Francki et al., 2000; Monchy et al., 2006). The pco/copABCD operon, transcribed from a copper-inducible promoter, is controlled by the two-component regulatory module pco/copRS (Mills et al., 1994). Although no copRS homologues were detected on pBTAi1, a two-component-system response regulator (BBta_3078/3079) similar to copRS was found on the Bradyrhizobium sp. BTAi1 chromosome, along with two copCD homologues (BBta_2513/4 and BBta_5430/1). This result suggests that transcription of copper-resistance genes is controlled by chromosomal regulators in Bradyrhizobium sp. BTAi1. Lim and Cooksey (1993) previously proposed that there is a linkage between chromosome- and plasmid-borne copper-resistance genes in P. syringae pv. tomato.
Plasmid-borne hydrogenase genes
Sequence analysis also revealed that pBTAi1 had a [Ni–Fe] hydrogenase gene cluster (ORFs BBta_p0170 to p0180) containing the large (hupL) and small (hupS) hydrogenase subunits, and various hup- and hyp-like maturation genes required for hydrogenase activity. Two additional hydrogenase gene clusters were previously shown to be localized to the Bradyrhizobium sp. BTAi1 chromosome (ORFs BBta_0458 to 0482, and ORFs BBta_1993 to 2012; Giraud et al., 2007). One of the chromosome-borne hydrogenase clusters (ORFs BBta_1993 to 2012), type II, had almost identical gene synteny and high sequence similarity to hydrogenase genes found in other Rhizobium and Bradyrhizobium sp. strains. The large and small subunits showed 99/97%, 92/96%, 90/84% and 91/87% AA sequence identity to hupS and hupL, respectively, from Bradyrhizobium sp. ORS278, B. japonicum USDA 110, R. leguminosarum bv. viciae and Rhodopseudomonas palustris BisB5, respectively. We refer to these ORFs as comprising the ‘typical hydrogenase genes (chrom type II). In contrast, a second ‘atypical’ chromosomal-borne hydrogenase gene cluster (ORFs BBta_0458 to 0482), chrom type I, was only distantly related to typical rhizobial hydrogenases, and shared much greater gene synteny and sequence similarity (81% and 84% AA sequence identity for the large and small hydrogenase subunits, respectively) to plasmid-borne genes from Sphingopyxis alaskensis strain RB2256, a heterotrophic bacterium prominent in oligotrophic marine environments. Phylogenetic analysis indicated that the atypical Bradyrhizobium. sp. BTAi1 and the S. alaskensis hydrogenase genes phylogenetically-cluster with cyanobacterial uptake hydrogenases from the genera Anabaena, Nostoc, Cyanothece and Nodularia (Figure 2).
Phylogeny of the three distinct Bradyrhizobium sp. BTAi1 uptake hydrogenase large subunit (hupL) genes relative to closely related reference species. Bootstrap values (100 replicates) are shown at branch nodes. Bar represents 10% sequence divergence. Phylogenetic trees were generated using the maximum likelihood method of the Phylip phylogenetic package (Felsenstein, 1989).
Sequence and synteny analyses revealed that the pBTAi1 plasmid-borne hydrogenase genes had a closer relationship to ORFs from the sulfur-oxidizing bacterium Thiobacillus denitrificans (Beller et al., 2006), than to either of the chromosomal-borne hydrogenase gene clusters in strain BTAi1 (Table 3; Figures 2 and 3). While the BTAi1 hupS and hupL had 67% and 57% AA sequence identity to the same genes in T. denitrificans, they only had only 54% and 45% AA identity to orthologues of the same genes in the ‘typical’ chromosomal-borne hydrogenase gene cluster in strain BTAi1. In addition, both the pBTAi1 and T. denitrificans hydrogenase clusters each lacked hupC, hupG, and hupI and hupJ, which are typically found in the hydrogenase gene clusters in other rhizobia (Baginsky et al., 2002). Despite this similarity, the pBTAi1 hydrogenase gene cluster did not contain the isp1 and isp2 genes that separate the large and small hydrogenase genes in the T. denitrificans (Figure 3).
Both the BTAi1 plasmid-borne (ORFs BBta_p0170 to p0180) and the atypical chromosomal-borne (BBta_0458 to 0482) hydrogenase genes were flanked by transposable elements, suggesting that they were acquired by means of lateral gene transfer from a non-rhizobial donor. Horizontal transfer of hydrogenase gene clusters between prokaryotes has been previously postulated (Calteau et al., 2005), and past indirect evidence suggested that hup sequences from specific Bradyrhizobium sp. (Vigna) strains may have been acquired via horizontal transfer from a non-rhizobial source (Baginsky et al., 2002).
Comparative expression of BTAi1 hydrogenase genes
Some rhizobia induce uptake hydrogenases during symbiotic growth in order to reduce energy losses associated with nitrogen fixation (Albrecht et al., 1979). The multiplicity of phylogenetically distinct uptake hydrogenases, and the presence of a plasmid-borne hydrogenase gene cluster in strain BTAi1, warranted assessment of the functional capacity of these enzymes and their potential role in symbiosis. Gas chromatographic analyses confirmed that both root and stems nodules of A. indica plants, inoculated with Bradyrhizobium sp. strain BTAi1, expressed hydrogenase activity (Figure 4). Quantitative real-time PCR analyses of mRNA from root and stem nodules, performed using specific primers targeting each of the three hupL genes, indicated that transcriptional levels of the two chromosome hupL homologues were significantly induced relative to free living BTAi1 cultures (Figure 5). In contrast, expression of the plasmid-borne hupL (BBta_p0179) in nodule extracts was considerably lower than the two chromosomal hupL homologues. While the small level of induction of the plasmid-encoded hupL in stem nodules suggests that plasmid-borne hydrogenase may be induced under symbiotic conditions, the relatively low level of expression implies that it does not play a significant role in the symbiotic process. Thus, the specific environmental role of this plasmid-borne hydrogenase still remains unclear.
In planta gene expression of the typical- (chrom type II), atypical-chromosomal (chrom type I) and plasmid-borne large hydrogenase subunits (hupL) of Bradyrhizbium sp. strain BTAi1 performed using quantitative real-time RT-PCR. Relative gene expression values for hupL under free-living and symbiotic (root and stem nodules) conditions were normalized to the parA housekeeping gene. Legend: ▪, root nodules; ( ), stem nodules.
), stem nodules.
Detection of accessory plasmids in diverse Bradyrhizobium sp. strains
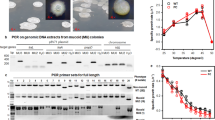
Reports of plasmids in other Bradyrhizobium strains have been inconsistent and infrequent, and results of these studies have been confusing due to changes in the taxonomy of soybean-nodulating bacteria. To determine whether the presence of accessory plasmids in the bradyrhizobia is relatively common, we used PFGE-CHEF to screen 46 Bradyrhizobium strains, isolated from the United States, China and Thailand. These strains were chosen due to their taxonomic and geographic diversity, and because many were isolated from the geographic origins of soybeans, China and Southeast Asia. In addition, many of the strains with USDA prefixes represent members of the main serogroups of B. japonicum and B. elkanii strains. Eleven of the tested strains (23.9%), from all three geographic locations, were found to harbor large, high-molecular-weight plasmids of varying sizes (75–330 kb; Figure 6). Sequence analysis of 16S rDNA indicated that these strains were either Bradyrhizobium elkani or B. japonicum (Table 4). Five strains of B. japonicum (USDA 135, USDA 146, USDA 177, USDA 225 and DASA01196) were found to possess plasmids, whereas six plasmid-containing strains (USDA 46, USDA 94, USDA 227, DSA01244, DSA011265 and DSA011304) were found to be B. elkanii. As has been previously reported in several other studies, the sequenced B. japonicum strain, USDA 110, was not found to contain any plasmids. Interestingly, to our knowledge, this is the first report of the presence of plasmids in B. elkanii strains.
Plasmids isolated from selected genetically and geographically diverse Bradyrhizobium sp. strains using pulse-field CHEF gel electrophoresis. Lanes: M, λ-PFG marker ladders; 1, B. elkanii USDA 46; 2, B. elkanii USDA 94; 3, B. japonicum USDA 135; 4, B. japonicum USDA 146 and 5, B. japonicum USDA 225. PFG, pulsed-field gel.
Among the plasmid-containing bradyrhizobia, three strains originated from the United States and four each from China and Thailand. Thus, there does not appear to be a relationship between geographic origin of the bradyrhizobia and the presence of plasmids. While the majority of the strains contained a single plasmid, two strains, Bradyrhizobium japonicum strains USDA 135 DASA01196, harbored three and two plasmids, respectively. Our results showing multiple accessory plasmids in some strains, obtained using a highly effective CHEF-PFGE technique, are in contrast to those reported by Masterson et al., 1982 in which only one plasmid was found in each of the strains they examined. However, compared with the fast-growing rhizobia, such as R. leguminosarum, which can harbor 2–11 different plasmids, the bradyrhizobia appear to contain relatively few plasmids. Plasmid size in the various strains was found to be highly heterogeneous, with only two strains (DASA01244 and DASA01265) containing plasmids of identical size (260 kb). None of the plasmids identified in these studies hybridized to 32P-labeled nolA, nodD2, nodD1YABC, nodSUIJ or nodZ; or hup (pLNJ1) gene probes from B. japonicum strain USDA 110. Taken together, these results indicate that accessory plasmids are more commonly present in the bradyrhizobia than was previously thought, but none contain symbiosis-related nod or hup genes.
Conclusions
While previous studies have reported the sequence and in-depth analysis of symbiotic and accessory plasmids in Rhizobium, Mesorhizobium and Sinorhizobium strains, this is the first report of comprehensive sequence and analysis of a plasmid from a Bradyrhizobium sp. strain. Here we also demonstrated the presence of heterogeneously sized plasmids in geographically and genetically diverse Bradyrhizobium. Moreover, we report for the first time the presence of plasmids in B. elkanii. Complete sequence analysis of plasmid pBTAi1 from one of these strains, Bradyrhizobium sp. BTAi1, revealed the presence of a repABC replication module and several conjugation and gene transfer mechanisms that are characteristic of other rhizobial plasmids and α-proteobacteria. In contrast, the majority of coding sequences on pBTAi1 were not significantly related to any sequences on previously described rhizobial plasmids or chromosomes. The most notable functional genes identified on pBTAi1 encoded for proteins involved in copper resistance and hydrogen oxidation. Enhanced copper resistance relative to the closely related, plasmid-free phototrophic Bradyrhizobium sp. ORS278 suggests that the plasmid-borne copper-resistance genes may provide this strain an environmental advantage in metal-rich environments under free-living conditions. Sequence analyses of the plasmid-borne hydrogenase genes indicated that they were likely acquired from a non-symbiotic organism via horizontal gene transfer. The low level of induction of pBTAi1-encoded hydrogenase genes during symbiotic growth with A. indica, relative to two additional chromosomal-borne hydrogenase enzymes, suggests that the plasmid-borne hydrogenase is not significantly involved in symbiotic growth. While the phylogenetic affiliation of the pBTAi1-borne uptake hydrogenease with an enzyme system from a non-rhizobial species suggests that it may have a role in free-living growth, this question remains currently unresolved.
References
Albrecht SL, Maier RJ, Hanus FJ, Russell SA, Emerich DW, Evans HJ . (1979). Hydrogenase in Rhizobium japonicum increases nitrogen fixation by nodulated soybeans. Science 203: 1255–1257.
Baginsky C, Brito B, Imperial J, Manuel Palacios J, Ruiz-Argüeso T . (2002). Diversity and evolution of hydrogenase systems in rhizobia. Appl Environ Microbiol 68: 4915–4924.
Barnett MJ, Fisher RF, Jones T, Komp C, Abola AP, Barloy-Hubler F et al. (2001). Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc Natl Acad Sci USA 14: 9883–9888.
Barton BM, Harding GP, Zuccarelli AJ . (1995). A general method for detecting and sizing large plasmids. Anal Biochem 226: 235–240.
Beller HR, Chain PS, Letain TE, Chakicherla A, Larimer FW, Richardson PM et al. (2006). The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitrificans. J Bacteriol 188: 1473–1488.
Belogurov AA, Delver EP, Agafonova OV, Belogurova NG, Lee LY, Kado CI . (2000). Antirestriction protein ard (type C) encoded by IncW plasmid psa has a high similarity to the ‘protein transport’ domain of TraC1 primase of promiscuous plasmid RP4. J Mol Biol 296: 969–977.
Bignell C, Thomas CM . (2001). The bacterial ParA–ParB partitioning proteins. J Biotechnol 13: 1–34.
Birren B, Lai E . (1993). Pulsed Field Gel Electrophoresis a Practical Approach. Academic Press Inc.: USA, ISBN 0-12-101290-5.
Brito B, Baginsky C, Palacios JM, Cabrera E, Ruiz-Argueso T, Imperial J . (2005). Biodiversity of uptake hydrogenase systems from legume endosymbiotic bacteria. Biochem Soc Trans 33: 33–35.
Calteau A, Gouy M, Perriere G . (2005). Horizontal transfer of two operons coding for hydrogenases between bacteria and archaea. J Mol Evol 60: 557–565.
Casalot L, Rousset M . (2001). Maturation of the [NiFe] hydrogenases. Trends Microbiol 9: 228–237.
Cevallos MA, Porta H, Izquierdo J, Tun-Garrido C, Garcia-de-los-Santos A, Davila G et al. (2002). Rhizobium etli CFN42 contains at least three plasmids of the repABC family: a structural and evolutionary analysis. Plasmid 48: 104–116.
Evans HJ, Harker AR, Papen H, Russell SA, Hanus FJ, Zuber M . (1987). Physiology, biochemistry, and genetics of the uptake hydrogenase in rhizobia. Annu Rev Microbiol 41: 335–361.
Felsenstein J . (1989). PHYLIP—phylogeny inference package (Version 3.2). Cladistics 5: 164–166.
Francki KT, Chang BJ, Mee BJ, Collignon PJ, Susai V, Keese PK . (2000). Identification of genes associated with copper tolerance in an adhesion-defective mutant of Aeromonas veronii biovar sobria. FEMS Immunol Med Microbiol 29: 115–121.
Giraud E, Fleischman D . (2004). Nitrogen-fixing symbiosis between photosynthetic bacteria and legumes. Photosynth Res 82: 115–130.
Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC et al. (2007). Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312.
Gonzalez V, Santamaria RI, Bustos P, Hernandez-Gonzalez I, Medrano-Soto A, Moreno-Hagelsieb G et al. (2006). The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci USA 7: 3834–3839.
Gottfert M, Rothlisberger S, Kundig C, Beck C, Marty R, Hennecke H . (2001). Potential symbiosis-specific genes uncovered by sequencing a 410-kilobase DNA region of the Bradyrhizobium japonicum chromosome. J Bacteriol 183: 1405–1412.
Gross DC, Vidaver AK, Klucas RV . (1979). Plasmids, biological properties and efficacy of nitrogen fixation in Rhizobium japonicum strains indigenous to alkaline soils. J Gen Microbiol 114: 257–266.
He X, Chang W, Pierce DL, Seib LO, Wagner J, Fuqua C . (2003). Quorum sensing in Rhizobium sp. strain NGR 234 regulates conjugal transfer (tra) gene expression and influences growth rate. J Bacteriol 185: 809–822.
Itoh Y, Watson JM, Haas D, Leisinger T . (1984). Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid 11: 206–220.
Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, Sasamoto S et al. (2002). Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA 110. DNA Res 9: 189–197.
Keyser HH, van Berkum P, Weber DF . (1982). A comparative study of the physiology of symbioses formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol 70: 1626–1630.
Lane DJ . (1991). 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds). Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons: New York, pp 115–175.
Li PL, Farrand SK . (2000). The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J Bacteriol 182: 179–188.
Lim CK, Cooksey DA . (1993). Characterization of chromosomal homologs of the plasmid-borne copper resistance operon of Pseudomonas syringae. J Bacteriol 175: 4492–4498.
Martínez E, Palacios R, Sánchez F . (1987). Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol 169: 2828–2834.
Masterson RV, Russel PR, Atherly AG . (1982). Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol 152: 928–931.
Mercado-Blanco J, Toro N . (1996). Plasmids in rhizobia: the role of nonsymbiotic plasmids. Mol Plant-Microb Interact 9: 535–545.
Mergeay M, Monchy S, Vallaeys T, Auquier V, Benotmane A, Bertin P et al. (1995). Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol Rev 27: 385–410.
Merrick MJ, Edwards RA . (1995). Nitrogen control in bacteria. Microbiol Rev 59: 604–622.
Mills SD, Lim CK, Cooksey DA . (1994). Purification and characterization of CopR, a transcriptional activator protein that binds to a conserved domain (cop box) in copper-inducible promoters of Pseudomonas syringae. Mol Gen Genet 15: 341–351.
Monchy S, Benotmane MA, Wattiez R, van Aelst S, Auquier V, Borremans B et al. (2006). Transcriptomic and proteomic analyses of the pMOL30-encoded copper resistance in Cupriavidus metallidurans strain CH34. Microbiology 152: 1765–1776.
Pappas KM, Winans SC . (2003). A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol 48: 1059–1073.
Paulsen IT, Seshadri R, Nelson KE, Eisen JA, Heidelberg JF, Read TD et al. (2002). The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc Natl Acad Sci USA 99: 13148–13153.
Ramirez-Romero M, Soberón N, Pérez-Oseguera A, Téllez-Sosa J, Cevallos MA . (2000). Structural elements required for replication and incompatibility of the Rhizobium etli symbiotic plasmid. J Bacteriol 182: 3117–31124.
Ramirez-Romero MA, Tellez-Sosa J, Barrios H, Perez-Oseguera A, Rosas V, Cevallos MA . (2001). RepA negatively autoregulates the transcription of the repABC operon of the Rhizobium etli symbiotic plasmid basic replicon. Mol Microbiol 42: 195–204.
Rensing C, Grass G . (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27: 197–213.
Rozen S, Skaletsky HJ . (2000). Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds). Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press: Totowa, NJ, pp 365–386.
Sadowsky MJ, Cregan PB, Göttfert M, Sharma A, Gerhold D, Rodriguez-Quinones F et al. (1991). The Bradyrhizobium japonicum nolA gene and its involvement in the genotype-specific nodulation of soybeans. Proc Natl Acad Sci USA 88: 637–641.
Sadowsky MJ, Kosslak RM, Madrzak CJ, Golinska B, Cregan PB . (1995). Restriction of nodulation by Bradyrhizobium japonicum is mediated by factors present in the roots of Glycine max. Appl Environ Microbiol 61: 832–836.
Sadowsky MJ, Tully RE, Cregan PB, Keyser HH . (1987). Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybeans. Appl Environ Microbiol 53: 2624–2630.
Sajjaphan K, Shapir N, Wackett LP, Palmer M, Blackmon B, Tomkins J et al. (2006). Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Escherichia coli. Appl Environ Microbiol 70: 4402–4407.
Sawada H, Kuykendall LD, Young JM . (2003). Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J Gen Appl Microbiol 49: 155–179.
Somasagaran P, Hoben H . (1994). Handbook for Rhizobium: Methods in Legume–Rhizobium Technology. Springer: Berlin Heidelberg New York.
Stiens M, Schneiker S, Keller M, Kuhn S, Puhler A, Schluter A . (2006). Sequence analysis of the 144-kilobase accessory plasmid pSmeSM11a, isolated from a dominant Sinorhizobium meliloti strain identified during a long-term field release experiment. Appl Environ Microbiol 72: 3662–3672.
Stowers MD, Eaglesham ARJ . (1983). A stem-nodulating Rhizobium with physiological characteristics of both fast and slow growers. J Gen Microbiol 129: 3651–3655.
Summerfield AS, Huxley PA, Minchin FR . (1977). Plant husbandry and management techniques for growing grain legumes under simulated conditions in controlled environment. Expl Tropical Agric 13: 81–92.
Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, Cruveiller S et al. (2006). MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res 34: 53–65.
van Berkum P, Leibold JM, Eardly BD . (2006). Proposal for combining Bradyrhizobium spp. (Aeschynomene indica) with Blastobacter denitrificans and to transfer Blastobacter denitrificans (Hirsch and Muller, 1985) to the genus Bradyrhizobium as Bradyrhizobium denitrificans (comb. nov.). Syst Appl Microbiol 29: 207–215.
Wang ET, van Berkum P, Sui XH, Beyene D, Chen WX, Martínez-Romero E . (1999). Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int J Syst Bacteriol 1: 51–65.
Watson RJ, Heys R . (2006). Replication regions of Sinorhizobium meliloti plasmids. Plasmid 55: 87–98.
Young JP, Crossman LC, Johnston AW, Thomson NR, Ghazoui ZF, Hull KH et al. (2006). The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol 7: R34; doi:10.1186/gb-2006-7-4-r34.
Acknowledgements
We thank John Ferguson for help with figures, Jimmy Gosse for technical assistance with GC analyses and Peter van Berkum and Achara Nuntagij for providing strains. This work was supported, in part, by Grant 2004-35604-14708 from USDA/CREES/NRI (to MJS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Cytryn, E., Jitacksorn, S., Giraud, E. et al. Insights learned from pBTAi1, a 229-kb accessory plasmid from Bradyrhizobium sp. strain BTAi1 and prevalence of accessory plasmids in other Bradyrhizobium sp. strains. ISME J 2, 158–170 (2008). https://doi.org/10.1038/ismej.2007.105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2007.105
Keywords
This article is cited by
-
Genome-wide TIFY family in Arachis hypogaea in the perspective of legume JAZs
Journal of Crop Science and Biotechnology (2022)
-
Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: elite model strains for understanding symbiotic performance with soybean
BMC Genomics (2014)
-
Complete genome and comparative analysis of the chemolithoautotrophic bacterium Oligotropha carboxidovorans OM5
BMC Genomics (2010)