Abstract
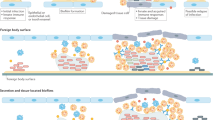
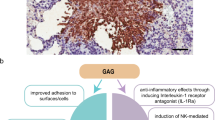
Bacteria survive in nature by forming biofilms on surfaces and probably most, if not all, bacteria (and fungi) are capable of forming biofilms. A biofilm is a structured consortium of bacteria embedded in a self‐produced polymer matrix consisting of polysaccharide, protein and extracellular DNA. Bacterial biofilms are resistant to antibiotics, disinfectant chemicals and to phagocytosis and other components of the innate and adaptive inflammatory defense system of the body. It is known, for example, that persistence of staphylococcal infections related to foreign bodies is due to biofilm formation. Likewise, chronic Pseudomonas aeruginosa lung infections in cystic fibrosis patients are caused by biofilm growing mucoid strains. Gradients of nutrients and oxygen exist from the top to the bottom of biofilms and the bacterial cells located in nutrient poor areas have decreased metabolic activity and increased doubling times. These more or less dormant cells are therefore responsible for some of the tolerance to antibiotics. Biofilm growth is associated with an increased level of mutations. Bacteria in biofilms communicate by means of molecules, which activates certain genes responsible for production of virulence factors and, to some extent, biofilm structure. This phenomenon is called quorum sensing and depends upon the concentration of the quorum sensing molecules in a certain niche, which depends on the number of the bacteria. Biofilms can be prevented by antibiotic prophylaxis or early aggressive antibiotic therapy and they can be treated by chronic suppressive antibiotic therapy. Promising strategies may include the use of compounds which can dissolve the biofilm matrix and quorum sensing inhibitors, which increases biofilm susceptibility to antibiotics and phagocytosis.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Høiby, N., Ciofu, O., Johansen, H. et al. The clinical impact of bacterial biofilms. Int J Oral Sci 3, 55–65 (2011). https://doi.org/10.4248/IJOS11026
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4248/IJOS11026
Keywords
This article is cited by
-
Oral microbiota analyses of paediatric Saudi population reveals signatures of dental caries
BMC Oral Health (2023)
-
Identification of molecular subgroups in osteomyelitis induced by staphylococcus aureus infection through gene expression profiles
BMC Medical Genomics (2023)
-
Osteosynthesis-associated infection in maxillofacial surgery by bacterial biofilms: a retrospective cohort study of 11 years
Clinical Oral Investigations (2023)
-
Electrochemical impedance response of the nanostructured Ti–6Al–4V surface in the presence of S. aureus and E. coli
Journal of Applied Electrochemistry (2023)
-
A comparative study of iron nanoflower and nanocube in terms of antibacterial properties
Applied Nanoscience (2023)