Abstract
Background:
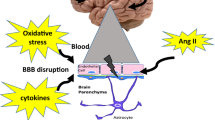
While vascular risk factors including Western-styled diet and obesity are reported to induce cognitive decline and increase dementia risk, recent reports consistently suggest that compromised integrity of cerebrovascular blood–brain barrier (BBB) may have an important role in neurodegeneration and cognitive deficits. A number of studies report that elevated blood pressure increases the permeability of BBB.
Methods:
In this study, we investigated the effects of antihypertensive agents, candesartan or ursodeoxycholic acid (UDCA), on BBB dysfunction and cognitive decline in wild-type mice maintained on high fat and fructose (HFF) diet for 24 weeks.
Results:
In HFF-fed mice, significantly increased body weight with elevated blood pressure, plasma insulin and glucose compared with mice fed with low-fat control chow was observed. Concomitantly, significant disruption of BBB and cognitive decline were evident in the HFF-fed obese mice. Hypertension was completely prevented by the coprovision of candesartan or UDCA in mice maintained on HFF diet, while only candesartan significantly reduced the body weight compared with HFF-fed mice. Nevertheless, BBB dysfunction and cognitive decline remained unaffected by candesartan or UDCA.
Conclusions:
These data conclusively indicate that modulation of blood pressure and/or body weight may not be directly associated with BBB dysfunction and cognitive deficits in Western diet-induced obese mice, and hence antihypertensive agents may not be effective in preventing BBB disruption and cognitive decline. The findings may provide important mechanistical insights to obesity-associated cognitive decline and its therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marseglia A, Fratiglioni L, Laukka EJ, Santoni G, Pedersen NL, Backman L et al. Early cognitive deficits in type 2 diabetes: a population-based study. J Alzheimers Dis 2016; 53: 1069–1078.
Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N et al. type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100000 cases of dementia. Diabetes Care 2016; 39: 300–307.
Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K et al. Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood–brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension 2012; 59: 1079–1088.
Stranahan AM, Hao S, Dey A, Yu X, Baban B . Blood–brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab 2016; 36: 2108–2121.
Mogi M, Horiuchi M . Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J 2011; 75: 1042–1048.
Agrawal RP, Ola V, Bishnoi P, Gothwal S, Sirohi P, Agrawal R . Prevalence of micro and macrovascular complications and their risk factors in type-2 diabetes mellitus. J Assoc Physicians India 2014; 62: 504–508.
Mohammadi MT, Dehghani GA . Acute hypertension induces brain injury and blood–brain barrier disruption through reduction of claudins mRNA expression in rat. Pathol Res Pract 2014; 210: 985–990.
Tayebati SK, Amenta F, Tomassoni D . Cerebrovascular and blood–brain barrier morphology in spontaneously hypertensive rats: effect of treatment with choline alphoscerate. CNS Neurol Disord Drug Targets 2015; 14: 421–429.
Kruyer A, Soplop N, Strickland S, Norris EH . Chronic hypertension leads to neurodegeneration in the TgSwDI mouse model of Alzheimer's disease. Hypertension 2015; 66: 175–182.
Al-Salami H, Mamo JC, Mooranian A, Negrulj R, Lam V, Elahy M et al. Long-term supplementation of microencapsulated ursodeoxycholic acid prevents hypertension in a mouse model of insulin resistance. Exp Clin Endocrinol Diabetes 2016; 125: 28–32.
Mooranian A, Negrulj R, Arfuso F, Al-Salami H . Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: formulation characterization and effects on production of insulin and inflammation in a pancreatic beta-cell line. Artif Cells Nanomed Biotechnol 2015; 44: 1642–1653.
Champagne D, Dupuy JB, Rochford J, Poirier J . Apolipoprotein E knockout mice display procedural deficits in the Morris water maze: analysis of learning strategies in three versions of the task. Neuroscience 2002; 114: 641–654.
Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC . Aging-related changes in blood–brain barrier integrity and the effect of dietary fat. Neurodegener Dis 2013; 12: 125–135.
Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC . Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood–brain barrier function in wild-type mice. J Neuroinflamm 2013; 10: 73.
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C et al. Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 2015; 12: 2.
Bian GL, Wei LC, Shi M, Wang YQ, Cao R, Chen LW . Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res 2007; 1150: 55–61.
Larochelle C, Alvarez JI, Prat A . How do immune cells overcome the blood–brain barrier in multiple sclerosis? FEBS Lett 2011; 585: 3770–3780.
Tsuge M, Yasui K, Ichiyawa T, Saito Y, Nagaoka Y, Yashiro M et al. Increase of tumor necrosis factor-alpha in the blood induces early activation of matrix metalloproteinase-9 in the brain. Microbiol Immunol 2010; 54: 417–424.
Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W et al. Tumour necrosis factor-alpha affects blood–brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int 2010; 30: 1198–1210.
Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P . Interleukin-1beta -induced changes in blood–brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 2000; 20: 8153–8159.
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR . VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc Natl Acad Sci USA 2009; 106: 1977–1982.
Suidan GL, Dickerson JW, Chen Y, McDole JR, Tripathi P, Pirko I et al. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J Immunol 2010; 184: 1031–1040.
Jin AY, Tuor UI, Rushforth D, Kaur J, Muller RN, Petterson JL et al. Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci 2010; 11: 12.
Dietrich JB . The adhesion molecule ICAM-1 and its regulation in relation with the blood–brain barrier. J Neuroimmunol 2002; 128: 58–68.
Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O et al. Reversible demyelination, blood–brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol 2004; 165: 1827–1837.
Ek CJ, D'Angelo B, Baburamani AA, Lehner C, Leverin AL, Smith PL et al. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia–ischemia. J Cereb Blood Flow Metab 2015; 35: 818–827.
Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF . Effects of acute hypoxia and hyperthermia on the permeability of the blood–brain barrier in adult rats. J Appl Physiol (1985) 2009; 107: 1348–1356.
Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N et al. Oxidative stress increases blood–brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 2010; 30: 1625–1636.
Allen CL, Bayraktutan U . Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood–brain barrier hyperpermeability. Diabetes Obes Metab 2009; 11: 480–490.
Kim YS, Seifert T, Brassard P, Rasmussen P, Vaag A, Nielsen HB et al. Impaired cerebral blood flow and oxygenation during exercise in type 2 diabetic patients. Physiol Rep 2015; 3: e12430.
Xia W, Rao H, Spaeth AM, Huang R, Tian S, Cai R et al. Blood pressure is associated with cerebral blood flow alterations in patients with T2DM as revealed by perfusion functional MRI. Medicine (Baltimore, MD) 2015; 94: e2231.
Takechi R, Galloway S, Pallebage-Gamarallage MM, Lam V, Mamo JC . Dietary fats, cerebrovasculature integrity and Alzheimer's disease risk. Prog Lipid Res 2010; 49: 159–170.
Kalaria RN . Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev 2010; 68 (Suppl 2): S74–S87.
Acknowledgements
We thank Curtin Health Innovation Research Institute for its technical and infrastructure support. We would also like to acknowledge Dr Mina Elahy for her assistance in sample and data collection. The study was financially supported by Western Australian Department of Health, Australian National Health and Medical Research Council Project Grants, Alzheimer’s Australia Dementia Research Foundation Project Grants and Curtin University Research Support Funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Mamo, J., Lam, V., Giles, C. et al. Antihypertensive agents do not prevent blood–brain barrier dysfunction and cognitive deficits in dietary-induced obese mice. Int J Obes 41, 926–934 (2017). https://doi.org/10.1038/ijo.2017.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.57
This article is cited by
-
Daily blood pressure profile and blood–brain barrier permeability in patients with cerebral small vessel disease
Scientific Reports (2022)
-
Blood–brain barrier disruption and ventricular enlargement are the earliest neuropathological changes in rats with repeated sub-concussive impacts over 2 weeks
Scientific Reports (2021)
-
Protein-bounded uremic toxin p-cresylsulfate induces vascular permeability alternations
Histochemistry and Cell Biology (2018)