Abstract
Background/Objectives:
The purpose of this study was to determine whether circulating pro-inflammatory cytokines, elevated with increased fat mass and ageing, were associated with muscle properties in young and older people with variable adiposity.
Subjects/Methods:
Seventy-five young (18–49 yrs) and 67 older (50–80 yrs) healthy, untrained men and women (BMI: 17–49 kg/m2) performed isometric and isokinetic plantar flexor maximum voluntary contractions (MVCs). Volume (Vm), fascicle pennation angle (FPA), and physiological cross-sectional area (PCSA) of the gastrocnemius medialis (GM) muscle were measured using ultrasonography. Voluntary muscle activation (VA) was assessed using electrical stimulation. GM specific force was calculated as GM fascicle force/PCSA. Percentage body fat (BF%), body fat mass (BFM), and lean mass (BLM) were assessed using dual-energy X-ray absorptiometry. Serum concentration of 12 cytokines was measured using multiplex luminometry.
Results:
Despite greater Vm, FPA, and PCSA (P<0.05), young individuals with BF% ⩾40 exhibited 37% less GM specific force compared to young BF%<40 (P<0.05). Older adults with BF% ⩾40 showed greater isokinetic MVC compared to older BF%<40 (P=0.019) but this was reversed when normalised to body mass (P<0.001). IL-6 correlated inversely with VA in young (r=−0.376; P=0.022) but not older adults (p>0.05), while IL-8 correlated with VA in older but not young adults (r⩾0.378, P⩽0.027). TNF-alpha correlated with MVC, lean mass, GM FPA and maximum force in older adults (r⩾0.458; P⩽0.048).
Conclusions:
The age- and adiposity-dependent relationships found here provide evidence that circulating pro-inflammatory cytokines may play different roles in muscle remodelling according to the age and adiposity of the individual.
Similar content being viewed by others
Introduction
Obesity, defined as a body mass index (BMI)⩾30, is a major global public health concern, with over 500 million people worldwide classified as overweight or obese.1 As well as being linked to metabolic disorders,2, 3, 4 the maximum muscle strength produced by obese individuals is significantly less than that generated by non-obese people when normalised to body mass,5 limb lean mass,6 or to the muscle physiological cross-sectional area (PCSA).7 This could lead to major functional limitations for daily living activities, such as climbing/descending stairs, rising from a chair, recovering from a trip, etc., which in turn could lead to hospitalisation and reduced quality of life.
There are a number of reasons why muscle quality (defined here as muscle specific force, i.e., the maximum force per unit PCSA) might be lower in obese vs non-obese people. Fat infiltration within the skeletal muscle reduces the contractile component of the total muscle volume,8 thereby lowering the intrinsic strength of the whole muscle.9 Furthermore, intramuscular lipid acts as a chemoattractant for macrophages,10 which produce pro-inflammatory cytokines,11 such as tumour necrosis factor-alpha (TNF-α)12 and interleukin-6 (IL-6).13 This chronic immune response is characterised by elevated concentrations of these pro-inflammatory cytokines in the blood resulting in chronic low-grade systemic inflammation, which has been observed in young12, 14 and older15 obese people. To compound this problem, both adipocytes16 and macrophages11 secrete pro-inflammatory cytokines, and it has been suggested that fat accounts for approximately 30% of serum IL-6 levels in humans.17 Thus, a chronically inflamed environment is created within and around the muscle containing substantial intramuscular fat. Crucially, as well as acting as chemoattractants, these cytokines are directly involved in the breakdown of muscle protein.18 This would interfere with the accretion of contractile material caused by the chronic low-intensity overloading of the muscle,19 thus reducing muscle specific force. However, it is unclear to what extent chronic inflammation is responsible for the reduction in muscle quality and muscle function in obese individuals.
As well as the increase in obesity incidence, we are living for longer. This is particularly relevant, as ageing is associated with a decrease in muscle activation capacity,20 loss of muscle size,21 a decrease in the fascicle pennation angle,22 i.e., the angle at which the fascicles insert into the lower aponeurosis [the smaller the muscle fibre CSA, the smaller the pennation angle23], and reduced muscle strength, both at the whole muscle and fascicle levels.21 Collectively, these physiological changes in muscle properties with age lead to senile sarcopaenia, which is thought to be a major factor underlying the incidence of falls and reduced quality of life in older people.24 Furthermore, ageing is associated with increased intramuscular fat,25, 26 which is likely a major cause of chronically elevated levels of pro-inflammatory circulating cytokines27, 28 and lower muscle specific force21 in older vs younger adults. Furthermore, central inflammation, i.e., elevated pro-inflammatory cytokines in the hippocampus and hypothalamus, leads to neuroinflammation,29 which might help explain the lower neuromuscular activation and absolute strength commonly observed in older vs younger individuals.6, 20, 21, 30 Moreover, as a high fat diet can elevate pro-inflammatory cytokine expression and activate the pro-inflammatory transcription factor nuclear factor-κB (NF-κB) in the hypothalamus,31 it is feasible that a higher fat diet in both young and older obese individuals could have reduced ability to activate their muscles compared to leaner persons due to neuroinflammation.
Although both obesity and ageing can lead to chronic low grade systemic inflammation, it is not known if systemic inflammation in obese older adults signifies a cumulative negative impact of both ageing and obesity on skeletal muscle properties. Therefore, the aim of this study was to determine whether circulating pro-inflammatory cytokines, elevated with increased fat mass12, 32, 33 and ageing,27, 28, 34 could explain the different effects of obesity on skeletal muscle size, architecture and strength between young and older adults. We hypothesised that pro-inflammatory cytokines would be associated with lower muscle size and strength in both young obese and older individuals. Further, we hypothesised that circulating pro-inflammatory cytokine concentration would be inversely correlated with neuromuscular activation and strength in older and obese people.
Subjects and Methods
Subjects
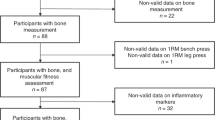
One hundred and forty-two healthy, untrained men and women gave their written informed consent to participate in this study, which complied with the Declaration of Helsinki35 and was approved by the local ethics committee of Manchester Metropolitan University. Participants were categorised according to total body fat percentage (normal: <40%; high: ⩾40% for females,7, 36, 37 and normal: <28%; high: ⩾28% for males36) and age (young: 18–49 yrs; older: 50–80 yrs), and their physical characteristics are shown in Table 1. Within the total cohort, there were 75 young (56 women, 22 men) and 67 older (48 women, 19 men) participants (Table 1). Of the younger group, 35 women and 17 men were normal BF%, and 19 women and 4 men were high BF%. Of the older group, 19 women and 4 men were normal BF%, and 29 women and 15 men were high BF%. Volunteers were screened for general health and habitual physical activity via questionnaire prior to participation. Exclusion criteria included history of lower limb muscle/tendon/joint disorders that affected mobility or the ability to exert maximum plantar-/dorsiflexor force; any chronic inflammatory condition; immunosuppressant medication; pregnancy; history of lower-limb resistance training in the six months prior to the start of the study; participation in >2 hourly sessions of structured physical activity a week; cognitive impediments.
Experimental design
Participants visited the laboratory on three separate occasions within 14 days. During the first session, participants provided a fasted 10 ml venous blood sample, from which serum levels of 12 inflammatory cytokines were quantified, and a sub-sample of participants were instructed how to use a 3-day food and drink diary, which was used to analyse habitual dietary fat intake. All 142 participants were subsequently familiarised to the muscle function assessments, which consisted of isometric and isokinetic plantar flexor (PF) and dorsiflexor (DF) maximum voluntary contractions (MVCs), and ramp isometric PF contractions to measure gastrocnemius medialis (GM) muscle architecture (fascicle length, Lf, and pennation angle, θp). On the second visit, participants completed a full body dual-energy X-ray absorptiometry (DXA) scan in the fasted state to assess total body composition. GM muscle volume was then determined from axial ultrasound scans and muscle length in 127 (men, n=32; women, n=95) participants. On the third and final visit, participants repeated the muscle function assessments, and the highest MVC scores from either the familiarisation or third laboratory session were used for subsequent analysis. The muscle function and morphology measurements were all performed on the dominant limb. Joint torque, joint angle, electromyographic (EMG) activity and electrical stimulation signals were interfaced with an analogue-to-digital converter (Biopac Systems Inc, Santa Barbara, USA), sampled at 2,000 Hz and displayed on the screen of an iMac computer (Apple, Cupertino, USA) using AcqKnowledge software (Biopac Systems).
Body composition
Participants a whole body DXA scan (Discovery W, Hologic Inc., Bedford, USA) between 08:00 and 09:00 after a 12 h fast. Total body fat mass, lean mass, and body fat percentage (BF%), were calculated using Hologic APEX software (version 3.3).
Dietary fat intake
Habitual dietary fat profiles of a subsample of 61 subjects were assessed using a three-day food diary recorded over two weekdays (Thursday and Friday) and one weekend day (Saturday). Subjects recorded their eating and drinking habits in as much detail as possible, e.g., time of meal, weight of food/ingredients in grams and volume of drink in mL, commercial brand names of food/ingredients and drink, any leftovers and cooking preparation methods. Dietary analysis was conducted using Nutritics software (version 1.8, Nutritics Ltd., Co. Dublin, Ireland). From the food analysis, dietary fat (absolute values and normalised to body mass) for each subject was calculated as an average over the three-day period.
Neuromuscular measurements
PF and DF MVCs
Participants sat on the chair of an isokinetic dynamometer (Cybex Norm, Cybex International, New York, USA) with a hip angle of 85° (180° corresponding to the supine position) and were firmly strapped at the hip, distal thigh and chest with inextensible straps to minimise extraneous movement. The dominant leg was fully extended and the foot was securely fastened to the dynamometer footplate with the lateral malleolus aligned with the axis of rotation. Participants performed 2–3 isometric PF and DF MVCs (iMVCs) for 2–3 s (alternating between PF and DF every 60 s) at each of the following ankle joint positions: −5° (DF), 0° (neutral position, foot 90° to tibia) and 10° (PF). DF iMVCs were performed to obtain maximum DF EMG, in order to calculate antagonist co-activation during PF iMVC (see below). Participants also performed three consecutive isokinetic PF MVCs (ikMVCs) at 60° s−1 from −5° to 10°.
Antagonist muscle co-activation
After appropriate skin preparation, two bipolar Ag-AgCl surface EMG electrodes (Neuroline, Medicotest, Rugmarken, Denmark) were placed 20 mm apart along the sagittal axis over the proximal third of the tibialis anterior (TA) muscle belly (SENIAM), with a reference electrode positioned over the lateral tibial condyle. The EMG signal was pre-amplified (× 2,000) and filtered using high- and low-pass filters set at 10 and 500 Hz, respectively (plus notch filter at 50 Hz). The root mean square (RMS) of the EMG signal was calculated over 1 s around the peak torque during each PF and DF MVC at all three joint angles. Thus, antagonist torque output during PF iMVC was calculated by dividing TA EMG RMS during PF iMVC by TA EMG RMS during DF iMVC, and multiplying DF iMVC torque by this ratio. The sum of the antagonist torque and PF iMVC torque was used to calculate maximum Achilles tendon force and GM muscle specific force (see below).
Voluntary muscle activation
The level of voluntary muscle activation was measured using the interpolated twitch technique (ITT) during the PF iMVCs with the joint set at 0°. Electrical muscle stimulation was administered percutaneously to the PF muscle group via two 5 × 10 cm self-adhesive electrodes (American Imex, Irvine, USA) placed distal to the popliteal crease (cathode) and the myotendinous junction of the soleus (anode). A supramaximal doublet (two twitch stimuli of 0.2 ms pulse width, administered at 100 Hz) was manually applied at rest (control doublet) 3 s before iMVC, and once during iMVC. The level of voluntary muscle activation was given by:

where t is the force of the superimposed doublet and T is the force of the control doublet. This percentage was used to calculate the maximum isometric torque able to be produced by the PF group (PF max torque) at each joint angle, as shown below:

Achilles tendon moment arm
The tendon excursion method using B-mode ultrasonography (AU5 Harmonic, Esaote Biomedica, Genoa, Italy), as described previously,38, 39 was used to determine the Achilles tendon moment arm. Participants were secured to the dynamometer chair and foot-plate (ankle set at 0°), as described above. A 2 mm wide, 2 cm long strip of surgical tape (3M, Neuss, Germany) was attached to the skin, transversely over the GM myotendinous junction (MTJ). The 4 cm wide, 7.5 MHz linear array ultrasound probe was then positioned sagitally over the tape to record the passive movement of the GM MTJ while the ankle was rotated between the angles of −5° and 10° at a constant velocity of 1° s−1. The ultrasound scan (including three continuous PF and DF rotations) was synchronised with the joint angle signal via a square wave signal generator. The displacement of the MTJ between 10° and −5° was measured with image analysis software (ImageJ, National Institutes of Health, Bethesda, USA) and the Achilles tendon moment arm at 0° was calculated as the MTJ displacement divided by change in the joint angle during a complete rotation (15°).
Achilles tendon force (Ft)
Ft was calculated by dividing PF max torque by the Achilles tendon moment arm.
Muscle volume
GM muscle volume (Vm) was measured with B-mode ultrasonography (AU5 Harmonic), using previously described methods validated in the vastus lateralis muscle.40 Participants lay relaxed in the prone position with the ankle angle set to 0°. The proximal end (0%) and 25, 50, 75 and 100% (distal end) of the GM muscle were located and marked on the skin. At 25, 50 and 75% muscle length, the lateral and medial boundaries were located and marked on the skin. The ultrasound probe was subsequently orientated in the axial plane, aligned perpendicular to the GM muscle, and moved along each pre-marked axial line at 25, 50 and 75% muscle length. Individual frames were exported and used to re-construct the anatomical cross-sectional areas (ACSAs) of the GM at 25, 50 and 75% muscle length using image-editing software (Photoshop, Adobe Systems Europe Ltd, Maidenhead, UK). Each of the three ACSAs was then measured using ImageJ. GM muscle volume (Vm) was then calculated by treating the muscle as a series of truncated cones.40 Each of the four truncated cones was calculated using the following equation, where d is the distance between two ACSAs (a and b):

The sum of the four cones provided the total GM Vm.
GM muscle architecture and PCSA
With the ankle joint set at 0°, the ultrasound probe was placed sagitally over the centre of the GM muscle, in line with the direction of the fascicles. The participant performed a ramped PF iMVC, gradually increasing torque over the course of 6 s. The frame depicting the GM muscle architecture at peak PF iMVC was exported and fascicle pennation angle (θp, the angle at which the fascicles insert into the lower aponeurosis) and length (Lf) were measured using ImageJ. The mean of the three θp and Lf measurements were subsequently used to calculate GM muscle fascicle force (GM Ff) and GM physiological cross-sectional area (PCSA), respectively (see below).
GM muscle fascicle force (Ff)
Based on the relative proportion of GM muscle volume to total PF muscle volume,41 the contribution of GM muscle to Ft was assumed to be 20.3%. Therefore, GM Ff was calculated by dividing Ft by the cosine of GM θp, as shown below:

GM muscle PCSA and specific force
GM PCSA at iMVC was calculated as GM Vm/Lf. Subsequently, dividing GM Ff by GM PCSA provided GM muscle specific force.
Serum inflammatory cytokine concentration
Participants provided a 10 ml fasted blood sample between 08:00 and 09:00, having performed no strenuous exercise for 48 h. Blood was collected in to an anticoagulant free vacutainer (BD Vacutainer Systems, Plymouth, UK) and serum was prepared and stored in 2 ml aliquots at −20 °C until subsequent analysis. Seventy-eight serum samples were randomly selected for cytokine analysis from a list of participant numbers with BMI and age as a guide for selection (the investigator was blinded to all other participant information). Consequently, 39 young and 39 older (n=48 BF%<40; n=31 BF%⩾40) participants were selected for analysis. Serum concentrations of eight inflammatory cytokines (pro-inflammatory: IL-1β, IL-6, TNF-α, G-CSF; anti-inflammatory: IL-10, TGF-β1, TGF-β2, TGF-β3) and four chemokines (IL-8, MCP-1, MIP-1α, MIP-1β) were measured using multiplex luminometry. A 3-plex panel was used to measure TGF-β1, β2 and β3 concentrations (R&D Systems Europe Ltd, Abingdon, UK) and a Bio-Plex Pro Human Inflammation Panel Assay (Bio-Rad laboratories Ltd., Hemel Hempstead, UK) was used to measure the remaining nine cytokines, following the manufacturer’s instructions. Samples were analysed using a Bio-Plex 200 system (Bio-Rad laboratories Ltd., Hemel Hempstead, UK).
Statistical analyses
IBM SPSS statistics (version 23, SPSS Inc., Chicago, IL) was used for all analyses. Two-way ANOVAs with Scheffe post hoc tests were used to determine the main effects of age, adiposity, and interaction for all of the study parameters. A Freidman’s ANOVA was used to compare between days differences in dietary fat content. Bivariate Pearson correlations were used to ascertain relationships between the serum concentration of individual inflammatory cytokines, and fat/muscle characteristics. Partial correlations (controlling for total body fat mass) were performed to determine the relationships between inflammatory cytokine concentration and muscle variables independently of fat mass. Partial correlations (controlling for age) were performed to determine the relationships between inflammatory cytokine concentration and muscle variables independently of age. Statistical significance was accepted when P⩽0.05. Data are presented as means±s.d. unless otherwise stated.
Results
Anthropometry, body composition
The younger individuals were taller (P<0.05), had a greater body mass (P<0.05) and greater lean mass (P<0.05) than the older participants (Table 1). There was no effect of adiposity on total body lean mass (P>0.05), although there was a tendency for the young high BF% group to have greater lean mass than the older high BF% group (ANOVA, age × adiposity, P=0.055). Young high BF% also presented with higher body mass, BMI and fat mass than the other three groups (P<0.05; Table 1). As expected, adiposity was positively associated with body mass, BMI and BF% (all, P<0.05) (Table 1).
Maximum strength
PF isometric and isokinetic MVC ankle joint torque
Regarding absolute iMVC, there was a main effect of age: young individuals produced higher PF iMVC compared to older persons (P<0.001; Table 1). There was also a main effect of adiposity, with high adipose persons being stronger than those of normal adiposity (P=0.007). However, there was no age × adiposity interaction (P=0.289; Table 1). Regarding absolute PF ikMVC, there was a main effect of age: young individuals produced higher ikMVC compared to older persons (P<0.001). There was no main effect of adiposity (P=0.128; Table 1) and no age × adiposity interaction (P=0.396; Table 1).
Maximum Achilles tendon force (Ft)
There was a main effect of age, i.e., young individuals produced greater Ft compared to older persons (P<0.001; Table 1), and a main effect of adiposity, with high adipose persons producing greater force than normal adipose individuals (P=0.020), but there was no age × adiposity interaction (P=0.472; Table 1).
Maximum GM fascicle force (Ff)
There was a main effect of age, i.e., younger individuals produced greater Ff than older persons (P<0.001; Table 1), and there was a main effect of adiposity (P=0.002), with high adipose persons producing more force than normal adipose individuals. However, there was no age × adiposity interaction (P=0.237; Table 1).
Maximum strength normalised to muscle size
After normalising iMVC and ikMVC to GM Vm, there were main effects for age on iMVC/Vm, i.e., older persons had lower iMVC/Vm and ikMVC/Vm than young individuals (P<0.05; Table 1), and there were main effects for adiposity (P<0.05; Table 1), with normal demonstrating greater muscle quality than high adipose persons. There were also age × adiposity interactions (P<0.05), i.e., young individuals with normal BF% had greater muscle quality compared to all other groups (P<0.05; Table 1). Regarding GM muscle specific force, there were no main effects of age or adiposity, or any interaction between age and adiposity (P>0.05; Table 1).
Voluntary muscle activation capacity
There were significant main effects for both age (P=0.001; Table 1) and adiposity (P=0.011; Table 1), demonstrating that young individuals had higher muscle activation capacity than older persons, and that persons with high BF% (regardless of age) had lower activation capacity than persons with normal BF%. There was no age × adiposity interaction (P=0.185; Table 1).
Muscle morphology
GM muscle volume and PCSA
There were main effects for age on GM Vm and PCSA: young persons had larger Vm and PCSA than older individuals (P<0.001; Table 1); and main effects for adiposity: persons with high BF% had larger Vm and PCSA than persons with normal BF% (P<0.001; Table 1). There were also age × adiposity interactions: young persons with high BF% had larger muscle volume and PCSA than all other groups (P<0.001; Table 1).
GM muscle architecture
There were main effects for both age (P<0.05; Table 1) and adiposity (P<0.05; Table 1), i.e., young persons had larger GM θp than older individuals, while persons with high BF% had larger θp than persons with normal BF% (P<0.05; Table 1). However, there was no age × adiposity interaction with GM θp (P>0.05). Further, there were no effects of age (P>0.05; Table 1) or adiposity (P>0.05; Table 1), and there was no age × adiposity interaction (P>0.05; Table 1) with GM fascicle length.
Serum inflammatory cytokine concentration
Associations with age and/or adiposity
Serum IL-6 concentration showed a significant main effect for age (P=0.004), with older participants having higher serum concentrations than young individuals (Table 2). There was no main effect of adiposity, and no interaction between age and adiposity (P>0.05). There was also an age × adiposity interaction (P=0.016) regarding serum MCP-1, with older individuals of high adiposity having higher levels than older normal adipose persons. Finally, there was a main effect of adiposity concerning TGF-β2 (P=0.008), with high adipose persons having a greater serum concentration of this cytokine than those of normal adiposity (Table 2).
Correlations with muscle and fat phenotypes in young and older persons combined
The positive and inverse relationships between serum IL-6, IL-1β, MCP-1, MIP-1α and MIP-1β concentrations and adiposity and muscle properties in young and older individuals combined are shown in Table 3.
Correlations with muscle and fat phenotypes in young persons only
The inverse correlation between serum IL-6 concentration and voluntary activation and, after controlling for BF%, the partial correlation between serum IL-6 and voluntary activation are shown in Table 4. Likewise, the inverse correlations between serum IL-1β and BMI, iMVC, ikMVC, fat mass, lean mass, GM PCSA, GM θp, (r⩾−0.325; P⩽0.049), and between serum MIP-1α and BF% (r=−0.343; P=0.035) and fat mass (r=−0.323; P=0.048) are displayed in Table 4. The positive correlations between serum MIP-1β and iMVC, GM Vm, GM Lf, Ft, GM PCSA, (r⩾0.339; P⩽0.040) and inverse correlation with ikMVC/Vm (r=−0.402; P=0.028) are also shown in Table 4.
Correlations with muscle and fat phenotypes in older persons only
Serum MCP1 correlated positively with BMI, BF% and fat mass (r⩾0.378; P⩽0.028; Table 4). Serum TNF-α correlated positively with iMVC, ikMVC, lean mass, GM θp, Ft, GM Ff, (r⩾0.458; P⩽0.048; Table 4). After controlling for total body fat mass, TNF-α correlated with iMVC, lean mass, GM θp, GM Ff, (r⩾0.510; P⩽0.044; Table 4). Serum IL-8 correlated positively with voluntary muscle activation (r=0.382; P=0.016; Table 4), and controlling for fat mass strengthened this correlation (r=0.414; P=0.010; Table 4).
Correlations with muscle and fat phenotypes in persons of normal and high adiposity
The positive and inverse relationships between serum IL-6, IL-1β, IL-8, TNF-α, MCP-1, MIP-1β, and G-CSF concentrations and adiposity and muscle properties in persons of normal and high adiposity are shown in Table 5. Most notably, IL-6 correlated inversely with on muscle quality in both normal and high adipose individuals (r⩾−0.367; P⩽0.042; Table 5), MIP-1β correlated with numerous measures of maximum muscle force and size in normal adipose persons (r⩾0.332; P⩽0.039; Table 5), and IL-8 correlated inversely with measures of muscle strength and quality in high adipose individuals (r⩾−0.364; P⩽0.044; Table 5), but positively with muscle quality in persons of normal adiposity (r=0.604; P=0.038; Table 5).
Habitual dietary fat intake
A Friedman’s ANOVA revealed no between days difference (P=0.448) between daily fat intake over the three-day dietary analysis period. For daily absolute fat intake, there was a main effect for age with young individuals consuming more fat than older persons (P=0.041; Table 6). There was no main effect for adiposity (P=0.088; Table 6) but there was an age × adiposity interaction, with young normal consuming more fat than all other groups (P<0.05) but otherwise no other differences (P>0.05). For daily fat intake normalised to body mass, there was no main effect for age (P>0.05) but there was a main effect for adiposity (P<0.001; Table 6), with normal consuming more fat than high. There was also an age × adiposity interaction (P=0.021; Table 6): young normal and old normal consumed per fat than both young high and old high. Concerning the daily amount of fat consumed as a percentage of total energy intake, there was no main effect of age (P=0.869) but there was a main effect for adiposity (P=0.043) and an age × adiposity interaction (P=0.025; Table 6), with normal young consuming a greater percentage of fat compared to high young (P=0.014). Finally, regarding the daily amount of fat consumed as a percentage of total macronutrient intake, there was no main effect of age (P=0.696) or adiposity (P=0.070), and no age × adiposity interaction (P=0.161; Table 6).
Correlations between habitual dietary fat content and cytokines
In young persons only, serum IL-1β (r=0.976; P=0.010) and IL-8 (r=0.879; P=0.021) correlated with absolute (g/day) fat intake. In older persons only, TGF-β3 correlated with fat intake, both in absolute terms (r=0.567; P=0.022) and normalised to body mass (r=0.509; P=0.044). In young and older individuals combined, TGF-β3 correlated positively with daily fat intake, both in absolute terms (r=0.561; P=0.015) and normalised to body mass (r=0.512; P=0.030).
Discussion
We aimed to determine whether circulating inflammatory cytokine levels could explain the different effects of obesity on skeletal muscle size, architecture and strength previously reported in young vs older adults. Our data suggest that, in young adults, serum IL-6 has a negative impact on neuromuscular activation, while IL-1β has a negative, and MIP-1β a positive, influence on muscle size, structure and strength (Table 4). In older adults, elevated IL-8 was positively associated with greater neuromuscular activation, while unexpectedly, TNF-α correlated positively with muscle mass, architecture and maximum strength (Table 4). In persons of normal adiposity, MIP-1β appears to play a very positive role in muscle size and strength, while IL-8 has a largely negative relationship with muscle quality (maximum strength normalised to either muscle size or body mass) in high adipose individuals (Table 5).
In line with previous research, we have shown that serum IL-6 concentration was higher in older vs young adults.27, 28 Furthermore, IL-6 correlated with BMI, total body fat mass and BF% in young and older individuals combined, which is in line with previous work.32 Moreover, the inverse relationship between IL-6 and voluntary muscle activation was particularly interesting given the positive relationships between IL-6 and adiposity reported here (Table 3) and elsewhere,12, 14, 15 and between IL-6 and ageing.27, 28 This inverse relationship (even after controlling for BF%) strongly suggests that IL-6 has a negative effect on the ability to voluntarily activate skeletal muscle. This may well be influenced by central inflammation, i.e., elevated levels of IL-6 and other pro-inflammatory cytokines in the hippocampus and hypothalamus (induced by a high fat diet31), which lead to neuroinflammation.29 This potentially reduces an individual’s ability to activate their muscles voluntarily, thus impairing muscle size and function. Not only might this help explain the significantly lower muscle activation and absolute strength values in older vs younger individuals reported here and elsewhere,6, 20, 21, 30 but it might also explain the lower muscle activation capacity we found here in young and older persons with high vs normal BF%. Although we did not find a relationship between IL-6 and habitual dietary fat intake, we did observe positive relations between fat intake and other pro-inflammatory cytokines (IL-1β and IL-8).
IL-8 is a chemokine that acts as a neutrophil chemotactic factor42 and is therefore pro-inflammatory. It has previously been associated with obesity,43 which coincides with our finding of higher (though non-significant) serum IL-8 levels in older persons with high BF% compared to older lean and younger adults. Surprisingly, we found that serum IL-8 correlated positively with voluntary muscle activation in older adults. As IL-8 is known to induce angiogenesis,42 enhance endothelial cell proliferation and have an anti-apoptotic effect,44 it is possible that this chemokine provides a protective, or rescuing, effect against neuroinflammation in older adults.
Both TNF-α mRNA content and TNF-α production are elevated in adipose tissue of obese individuals,12 while circulating TNF-α concentration also rises with obesity.45 We found a non-significant trend for serum TNF-α to correlate inversely with iMVC, and a significant inverse correlation between serum IL-1β and muscle size and strength and adiposity in young adults. In contrast, TNF-α correlated positively with iMVC, ikMVC, lean mass, GM θp, maximum tendon force and GM fascicle force in older adults, thus suggesting age-dependent effects of TNF-α on skeletal muscle characteristics. After controlling for fat mass, TNF-α remained positively correlated with older muscle size and strength. This indicates the effect of TNF-α was not due to fat mass per se, which is supported by TNF-α being produced almost exclusively by macrophages within adipose tissue, as opposed to the adipocytes themselves.11 Skeletal muscle also produces TNF-α46 and, although it has catabolic properties (thus possibly explaining the inverse correlation with iMVC in young adults), it has been shown to stimulate protein synthesis47 and positively influence maximum strength48 during the various phases of muscle remodelling. Furthermore, TNF-α induces IL-6 production in myoblasts49 and IL-6, in combination with TNF-α, stimulates myoblast growth.50 Therefore, it is feasible that the muscles of those older individuals with elevated serum TNF-α produced more IL-6, thus having a beneficial rather than deleterious effect on muscle size and strength.
In contrast to our TNF-α results, serum MIP-1β, correlated positively with muscle size, architecture and absolute strength in young adults, and with numerous measures of muscle size, structure and strength in persons of normal adiposity. MIP-1β is a chemoattractant for monocytes51 and interacts with MIP-1α,52 which correlated inversely with adiposity in our young participants. Thus, it appears that these chemokines have a positive effect on skeletal muscle properties in young but not in older muscle, and may therefore explain the previously reported lower muscle quality in older vs young adults.21
Our study has highlighted novel relationships between numerous circulating cytokines and precise measures of neuromuscular properties in young and older individuals of varied adiposity. These relationships indicate potential mechanisms that may explain the lower muscle quality in obese vs normal weight individuals,7 and in older vs younger persons,21 seen here and elsewhere. However, we acknowledge certain limitations with our study; as cognitive function was not a focus of this study (although our selection criteria excluded cognitively challenged volunteers), it is not possible to make a direct comparison with those previous studies that associated high levels of IL-6 with cognitive decline29). Furthermore, contrary to our hypothesis based on previous work,31 our data did not exhibit any association between habitual dietary fat intake and circulating IL-6 (although we did observe positive correlations between fat intake and IL-β1 and IL-8 in young persons). Nevertheless, our reported inverse relationship between IL-6 and voluntary muscle activation in young individuals is as expected in a physiological system linking high IL-6 with poor neuronal integration. Future human studies should consider these parameters (cognition, IL-6 endocrine profile, and diet) longitudinally. We would also recommend that studies employing an animal model may wish to build on previous work,31 by characterising neurodegeneration and muscle size and strength in young vs old animals fed with a high vs low fat diet. In older persons, we found that IL-8 correlated positively with voluntary activation but, to our knowledge, there is no evidence linking IL-8 with neurogenesis or repair following damage due to ageing. Future studies may wish to investigate this potential protective mechanism against neurodegeneration in older persons by measuring the effect of high vs low doses of IL-8 on damaged neurons in vitro and on neurological properties in an animal model in vivo. Finally, our somewhat surprising relationships between TNF-α and muscle size and strength in older persons only may be explained by TNF-α acting in a dose-response manner to reduce the rate of sarcopenia. Indeed in cell culture models, a concentration of this cytokine (as well as dependent on the order in which various cytokines are introduced in the culture dish), the effects of TNF-α can either induce hypertrophy or alternatively, muscle breakdown.50, 53
To conclude, our data suggest that obesity has a greater anabolic effect on young compared to older human muscle, but that it attenuates muscle quality more in young compared to older adults. The inverse relationship between serum IL-6 and voluntary muscle activation in young adults, and the chronically elevated levels of IL-6 and lower voluntary muscle activation levels in older adults, suggest that IL-6-induced neuroinflammation plays a role in reducing voluntary muscle strength. Interestingly, the unexpected positive relationship between IL-8 and voluntary muscle activation in older adults suggests this chemokine may be differentially impacting neuroinflammation and hence neuromuscular activation. The positive relationship between TNF-α and muscle mass and strength in older adults in the current cohort, whilst potentially supporting previous reports of TNF-α having a positive effect on skeletal muscle properties, is yet to be fully understood given the weight of the previous published evidence pertaining to the contrary (i.e., a deleterious muscle protein synthesis and/or stimulatory impact on the rate of protein degradation). Collectively, these age- and adiposity-dependent relationships provide evidence that circulating pro-inflammatory cytokines play different roles in neuromuscular remodelling according to the age and adiposity of the individual.
References
WHO Obesity and Overweight Fact Sheet No. 311. World Health Organization Media Centre: Geneva, Switzerland, 2013.
Kahn SE, Hull RL, Utzschneider KM . Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846.
Lavie CJ, Milani RV, Ventura HO . Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932.
Samuel VT, Shulman GI . Mechanisms for insulin resistance: common threads and missing links. Cell 2012; 148: 852–871.
Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A et al. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol 2007; 101: 51–59.
Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambele-Pearson GL . Combined effects of body composition and ageing on joint torque, muscle activation and co-contraction in sedentary women. Age (Dordrecht, Netherlands) 2014; 36: 9652.
Tomlinson DJ, Erskine RM, Winwood K, Morse CI, Onambele GL . Obesity decreases both whole muscle and fascicle strength in young females but only exacerbates the aging-related whole muscle level asthenia. Physiological reports 2014; 2: e12030.
Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR . Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 2008; 88: 1336–1344.
Rahemi H, Nigam N, Wakeling JM . The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. Journal of the Royal Society, Interface / the Royal Society 2015; 12: 20150365.
Kewalramani G, Bilan PJ, Klip A . Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care 2010; 13: 382–390.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM . Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415.
Park HS, Park JY, Yu R . Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 2005; 69: 29–35.
Valle M, Martos R, Gascon F, Canete R, Zafra MA, Morales R . Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab 2005; 31: 55–62.
Beavers KM, Beavers DP, Newman JJ, Anderson AM, Loeser RF Jr ., Nicklas BJ et al. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society 2014; 23: 249–256.
Fain JN . Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitamins and hormones 2006; 74: 443–477.
Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 1997; 82: 4196–4200.
Hayden MS, Ghosh S . NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 2012; 26: 203–234.
Johnson MA, Polgar J, Weightman D, Appleton D . Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 1973; 18: 111–129.
Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV . Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol 2004; 92: 219–226.
Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV . In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol 2005; 99: 1050–1055.
Morse CI, Thom JM, Birch KM, Narici MV . Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 2005; 183: 291–298.
Degens H, Erskine RM, Morse CI . Disproportionate changes in skeletal muscle strength and size with resistance training and ageing. J Musculoskelet Neuronal Interact 2009; 9: 123–129.
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clinical nutrition (Edinburgh, Scotland) 2012; 31: 652–658.
Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ . Cross-sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci 1995; 50: M307–M316.
Kehayias JJ, Fiatarone MA, Zhuang H, Roubenoff R . Total body potassium and body fat: relevance to aging. Am J Clin Nutr 1997; 66: 904–910.
Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J . Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiology of aging 1994; 15: 771–772.
Wei J, Xu H, Davies JL, Hemmings GP . Increase of plasma IL-6 concentration with age in healthy subjects. Life sciences 1992; 51: 1953–1956.
Miller AA, Spencer SJ . Obesity and neuroinflammation: A pathway to cognitive impairment. Brain, behavior, and immunity 2014; 42: 10–21.
Onambele GL, Narici MV, Maganaris CN . Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 2006; 100: 2048–2056.
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005; 146: 4192–4199.
Fried SK, Bunkin DA, Greenberg AS . Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998; 83: 847–850.
Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F et al. Sarcopenic obesity and inflammation in the InCHIANTI study. Journal of applied physiology (Bethesda, Md. : 1985) 2007; 102: 919–925.
Bartlett DB, Firth CM, Phillips AC, Moss P, Baylis D, Syddall H et al. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 2012; 11: 912–915.
WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 2013; 310: 2191–2194.
Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE . Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obesity research 2004; 12: 1995–2004.
Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr 2009; 89: 1895–1900.
Maganaris CN, Baltzopoulos V, Sargeant AJ . In vivo measurement-based estimations of the human Achilles tendon moment arm. Eur J Appl Physiol 2000; 83: 363–369.
Fath F, Blazevich AJ, Waugh CM, Miller SC, Korff T . Direct comparison of in vivo Achilles tendon moment arms obtained from ultrasound and MR scans. J Appl Physiol 2010; 109: 1644–1652.
Reeves ND, Narici MV, Maganaris CN . Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol 2004; 96: 885–892.
Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Edgerton VR . Specific tension of human plantar flexors and dorsiflexors. J Appl Physiol 1996; 80: 158–165.
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992; 258: 1798–1801.
Sharabiani MT, Vermeulen R, Scoccianti C, Hosnijeh FS, Minelli L, Sacerdote C et al. Immunologic profile of excessive body weight. Biomarkers 2011; 16: 243–251.
Li A, Dubey S, Varney ML, Dave BJ, Singh RK . IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 2003; 170: 3369–3376.
Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism 1999; 48: 1332–1335.
Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA . The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest 1996; 97: 1111–1116.
Plaisance I, Morandi C, Murigande C, Brink M . TNF-alpha increases protein content in C2C12 and primary myotubes by enhancing protein translation via the TNF-R1, PI3K, and MEK. Am J Physiol Endocrinol Metab 2008; 294: E241–E250.
Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI et al. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. Faseb j 2002; 16: 1630–1632.
Tseng WP, Su CM, Tang CH . FAK activation is required for TNF-alpha-induced IL-6 production in myoblasts. J Cell Physiol 2010; 223: 389–396.
Al-Shanti N, Saini A, Faulkner SH, Stewart CE . Beneficial synergistic interactions of TNF-alpha and IL-6 in C2 skeletal myoblasts—potential cross-talk with IGF system. Growth Factors 2008; 26: 61–73.
Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG . B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol 2001; 2: 1126–1132.
Guan E, Wang J, Norcross MA . Identification of human macrophage inflammatory proteins 1alpha and 1beta as a native secreted heterodimer. J Biol Chem 2001; 276: 12404–12409.
Saini A, Al-Shanti N, Faulkner SH, Stewart CE . Pro- and anti-apoptotic roles for IGF-I in TNF-alpha-induced apoptosis: a MAP kinase mediated mechanism. Growth Factors 2008; 26: 239–253.
Acknowledgements
Financial support for this study was received from the Centre for Health, Exercise and Active Living, Manchester Metropolitan University, and from a Liverpool John Moores University Fellowship Award, supporting inter-institution collaboration.
Author contributions
All muscle/adipose phenotype data and blood samples were collected in the physiology laboratories at Manchester Metropolitan University, Cheshire. The luminometry analyses were completed in the Institute of Inflammation and Ageing, University of Birmingham. RE, GO, CM, KW and DT contributed towards the design of the study; RE, GO, CM, KW, DT, PH, JL contributed towards the acquisition, analysis, or interpretation of data for the study; RE, GO, CM, KW, DT, PH, JL contributed towards the writing of the manuscript. All authors have approved the final version of the manuscript and agree (i) to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; (ii) that all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Erskine, R., Tomlinson, D., Morse, C. et al. The individual and combined effects of obesity- and ageing-induced systemic inflammation on human skeletal muscle properties. Int J Obes 41, 102–111 (2017). https://doi.org/10.1038/ijo.2016.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.151
This article is cited by
-
Comparison of gluteus medius strength between individuals with obesity and normal-weight individuals: a cross-sectional study
BMC Musculoskeletal Disorders (2021)
-
The combined effects of obesity and ageing on skeletal muscle function and tendon properties in vivo in men
Endocrine (2021)
-
Cord blood levels of interleukin-10 decrease in neonates with increased birth weight: novel implications of the cytokine network in early obesity
European Journal of Pediatrics (2021)
-
Problematic drinking in the old and its association with muscle mass and muscle function in type II diabetes
Scientific Reports (2019)
-
A high-fat diet impacts memory and gene expression of the head in mated female Drosophila melanogaster
Journal of Comparative Physiology B (2019)