Abstract
Background/Objectives:
Obese adipose tissue, especially the visceral depot, exhibits altered production of several molecules that could have a role on the initiation/promotion of breast cancer development. The aim of this work was to evaluate the effect of excess adipose tissue and its secreted factors on the expression of genes involved in the early steps of tumor promotion on the mammary gland.
Subjects and methods:
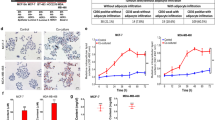
Carcinogenesis-related gene expression was evaluated in mammary gland tissue from female diet-induced obese (DIO) Sprague–Dawley rats and circulating leukocytes isolated from a group of breast cancer diagnosed and non-diagnosed obese women and compared with their normal weight counterparts. In addition, the human non-tumoral mammary epithelial cell line MCF10A was treated in vitro with the visceral (retroperitoneal adipose tissue (RPAT)) or subcutaneous adipose tissue (SAT) secretome and with rising concentrations of the lipid peroxidation by-product 4-hydroxynonenal (4-HNE).
Results:
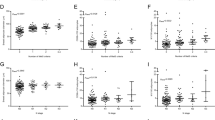
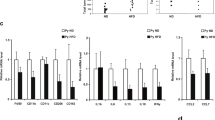
DIO rats were classified as susceptible to DIO (DIO-S) or partially resistant to DIO (DIO-R) according to the maximum fat mass gain of the lean group as a cut-off. As compared with lean and DIO-R, the DIO-S group showed a higher fat mass and lower lean mass. The anatomical characteristic of DIO-S was correlated with differential expression of cellular proliferation (ALDH3A1 and MYC) and antioxidant and DNA protection (GSTM2, SIRT1), and tumor suppression (TP53, PTEN, TGFB1) genes. Remarkably, this carcinogenesis-related gene expression pattern was reproduced in MCF10A treated with the RPAT secretome from DIO-S rats and with the lipid peroxidation by-product 4-HNE. Moreover, this pattern was also detected in leukocytes from obese women compared with normal weight women without evidence of breast cancer.
Conclusions:
Lipid peroxides secreted by the obese visceral adipose tissue could be among the relevant factors that promote changes involved in the early steps of tumor development in mammary gland. These changes can be detected even before histological alterations and in circulating leukocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377: 557–567.
Calle EE, Kaaks R . Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579–591.
Khandekar MJ, Cohen P, Spiegelman BM . Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011; 11: 886–895.
Lorincz AM, Sukumar S . Molecular links between obesity and breast cancer. Endocr Relat cancer 2006; 13: 279–292.
Stark A, Stahl MS, Kirchner HL, Krum S, Prichard J, Evans J . Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: findings from a case-series study. Int J Obes (Lond) 2010; 34: 1381–1386.
Crujeiras AB, Cueva J, Vieito M, Curiel T, Lopez-Lopez R, Pollan M et al. Association of breast cancer and obesity in a homogeneous population from Spain. J Endocrinol Invest 2012; 35: 681–685.
Abrahamson PE, Gammon MD, Lund MJ, Flagg EW, Porter PL, Stevens J et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev 2006; 15: 1871–1877.
Carmichael AR . Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG 2006; 113: 1160–1166.
Deglise C, Bouchardy C, Burri M, Usel M, Neyroud-Caspar I, Vlastos G et al. Impact of obesity on diagnosis and treatment of breast cancer. Breast Cancer Res Treat 2010; 120: 185–193.
Khan S, Shukla S, Sinha S, Meeran SM . Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev 2013; 24: 503–513.
Matafome P, Santos-Silva D, Sena CM, Seica R . Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab Res Rev 2013; 29: 285–295.
Hursting SD, Berger NA . Energy balance, host-related factors, and cancer progression. J Clin Oncol 2010; 28: 4058–4065.
Crujeiras AB, Diaz-Lagares A, Carreira MC, Amil M, Casanueva FF . Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res 2013; 47: 243–256.
Fang J, Seki T, Maeda H . Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 2009; 61: 290–302.
Gnerlich JL, Yao KA, Fitchev PS, Goldschmidt RA, Bond MC, Cornwell M et al. Peritumoral expression of adipokines and fatty acids in breast cancer. Ann Surg Oncol 2013; 20 (Suppl 3): S731–S738.
Santander AM, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E et al. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: the role of obesity and inflammation in breast adipose tissue. Cancers 2015; 7: 143–178.
Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V et al. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012; 2012: 789174.
Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R . Diabetes and cancer. Endocr Relat cancer 2009; 16: 1103–1123.
Shacter E, Weitzman SA . Chronic inflammation and cancer. Oncology (Williston Park) 2002; 16: 217–226 229; discussion 230-2.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752.
Hynes NE, Stoelzle T . Key signalling nodes in mammary gland development and cancer: Myc. Breast Cancer Res 2009; 11: 210.
Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA . Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med 2012; 52: 735–746.
Waligorska-Stachura J, Jankowska A, Wasko R, Liebert W, Biczysko M, Czarnywojtek A et al. Survivin—prognostic tumor biomarker in human neoplasms—review. Ginekol Pol 2012; 83: 537–540.
Rahman S, Islam R . Mammalian Sirt1: insights on its biological functions. Cell Commun Signal 2008; 9: 11.
Zhou SG, Wang P, Pi RB, Gao J, Fu JJ, Fang J et al. Reduced expression of GSTM2 and increased oxidative stress in spontaneously hypertensive rat. Mol Cell Biochem 2008; 309: 99–107.
Cho Y, Gorina S, Jeffrey PD, Pavletich NP . Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 1994; 265: 346–355.
Jakowlew SB . Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev 2006; 25: 435–457.
Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 2012; 151: 1185–1199.
Yin Y, Shen WH . PTEN: a new guardian of the genome. Oncogene 2008; 27: 5443–5453.
Crujeiras AB, Parra D, Goyenechea E, Martinez JA . Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest 2008; 38: 672–678.
Roca-Rivada A, Alonso J, Al-Massadi O, Castelao C, Peinado JR, Seoane LM et al. Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. J Proteomics 2013; 74: 1068–1079.
Ton CC, Vartanian N, Chai X, Lin MG, Yuan X, Malone KE et al. Gene expression array testing of FFPE archival breast tumor samples: an optimized protocol for WG-DASL sample preparation. Breast Cancer Res Treat 2011; 125: 879–883.
Crujeiras AB, Parra D, Milagro FI, Goyenechea E, Larrarte E, Margareto J et al. Differential expression of oxidative stress and inflammation related genes in peripheral blood mononuclear cells in response to a low-calorie diet: a nutrigenomics study. OMICS 2008; 12: 251–261.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Levin BE, Dunn-Meynell AA . Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 2000; 278: R231–R237.
Mattson MP . Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol 2009; 44: 625–633.
Vincent HK, Taylor AG . Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006; 30: 400–418.
Stemmer K, Perez-Tilve D, Ananthakrishnan G, Bort A, Seeley RJ, Tschop MH et al. High-fat-diet-induced obesity causes an inflammatory and tumor-promoting microenvironment in the rat kidney. Dis Model Mech 2012; 5: 627–635.
Black W, Chen Y, Matsumoto A, Thompson DC, Lassen N, Pappa A et al. Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal. Free Radic Biol Med 2012; 52: 1937–1944.
Beltran-Ramirez O, Sokol S, Le-Berre V, Francois JM, Villa-Trevino S . An approach to the study of gene expression in hepatocarcinogenesis initiation. Transl Oncol 2010; 3: 142–148.
Salminen A, Kaarniranta K, Kauppinen A . Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 2013; 14: 3834–3859.
Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N Jr et al. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol Pharmacol 2011; 79: 941–952.
Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development 2002; 129: 4159–4170.
Sieri S, Chiodini P, Agnoli C, Pala V, Berrino F, Trichopoulou A et al. Dietary fat intake and development of specific breast cancer subtypes. J Natl Cancer Inst 2014; 106: pii: dju068.
Fornari R, Francomano D, Greco EA, Marocco C, Lubrano C, Wannenes F et al. Lean mass in obese adult subjects correlates with higher levels of vitamin D, insulin sensitivity and lower inflammation. J Endocrinol Invest 2014; 38: 367–372.
Pedersen BK, Febbraio MA . Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–465.
Roca-Rivada A, Al-Massadi O, Castelao C, Senin LL, Alonso J, Seoane LM et al. Muscle tissue as an endocrine organ: comparative secretome profiling of slow-oxidative and fast-glycolytic rat muscle explants and its variation with exercise. J Proteomics 2012; 75: 5414–5425.
Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK . Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011; 2011: 490650.
Chandramathi S, Suresh K, Anita ZB, Kuppusamy UR . Comparative assessment of urinary oxidative indices in breast and colorectal cancer patients. J Cancer Res Clin Oncol 2009; 135: 319–323.
Huang YL, Sheu JY, Lin TH . Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem 1999; 32: 131–136.
Crujeiras AB, Parra D, Abete I, Martinez JA . A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res 2007; 41: 498–506.
Acknowledgements
This work was supported by grants from the Fondo de Investigacion Sanitaria, INTRASALUD programme (PI10/02464), PI14/01012 research projects, and CIBERobn (CB06/003), Instituto de Salud Carlos III (ISCIII)-Subdireccion General de Evaluacion y Fomento de la Investigacion; Fondo Europeo de Desarrollo Regional (FEDER), and the Health Department of the Government Xunta de Galicia (GRC2014/034), Spain as well as Fundacion Lilly and Fundacion Mapfre. B Cabia was funded by a Santiago de Compostela University (USC)-Campus Vida predoctoral contract (ref. 011–020). AB Crujeiras was funded by the ISCIII through a research contract ‘Sara Borrell’ (C09/00365). We thank Patricia Viaño for their excellent technical support in develop the hematoxylin and eosin staining and Dr Tomas García-Caballero for his advise and encouragement in this study, as well as the patients who voluntarily took part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Crujeiras, A., Cabia, B., Carreira, M. et al. Secreted factors derived from obese visceral adipose tissue regulate the expression of breast malignant transformation genes. Int J Obes 40, 514–523 (2016). https://doi.org/10.1038/ijo.2015.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.208
This article is cited by
-
Weight loss normalizes enhanced expression of the oncogene survivin in visceral adipose tissue and blood leukocytes from individuals with obesity
International Journal of Obesity (2021)
-
Ketogenic diets as treatment of obesity and type 2 diabetes mellitus
Reviews in Endocrine and Metabolic Disorders (2020)
-
Dual-Energy X-Ray Absorptiometry Compared to Computed Tomography for Visceral Adiposity Assessment Among Gastrointestinal and Pancreatic Cancer Survivors
Scientific Reports (2019)
-
The most important questions in cancer research and clinical oncology—Question 2–5. Obesity-related cancers: more questions than answers
Chinese Journal of Cancer (2017)
-
Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress?
International Journal of Obesity (2017)