Abstract
Background/Objectives:
It has been reported that irisin regulated exercise-mediated adipocyte browning; however, the systematical effects of irisin on the metabolism of glucose and lipid in diabetes are largely unknown. In the present study, we investigated the role and underlying mechanism of irisin in glucose utilization and lipid metabolism in diabetic mice.
Methods:
A mouse model of diabetes was established by feeding C57BL/6 mice with high-fat diet. The diabetic mice were then treated with irisin. To mimic type 2 diabetes in vitro, myocytes and hepatocytes were cultured in a medium of high glucose and high fat. Glucose uptake, fatty acid oxidation and the expression of related protein were evaluated.
Results:
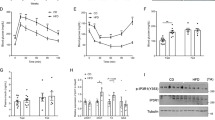
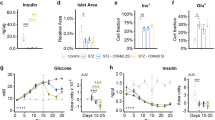
Irisin improved glucose tolerance and glucose uptake as evidenced by increased 18F-FDG accumulation and GLUT4 translocation in diabetic skeletal muscle. Irisin also increased glucose uptake in myocytes cultured in high glucose/high fatty acid medium. In contrast, irisin reduced the expression of PEPCK and G6Pase, which are involved in gluconeogenesis, in diabetic liver. Consistently, irisin reduced fat weight and serum total cholesterol and triglyceride levels in diabetic mice, but increased acetyl coenzyme A carboxylase-β phosphorylation in muscle tissue and uncoupling protein 1 expression in fat tissue. In addition, irisin increased the oxidation of fatty acid in myocytes. Knockdown of the adenosine monophosphate (AMP)-activated protein kinase (AMPK) attenuated the effects of irisin on glucose uptake and fatty acid β-oxidation in myocytes. Similarly, inhibition of AMPK by a specific inhibitor reduced the effects of irisin on PEPCK and G6Pase expression in hepatocytes.
Conclusions:
Our results suggest that irisin has an essential role in glucose utilization and lipid metabolism, and irisin is a promising pharmacological target for the treatment of diabetes and its complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kahn BB, Flier JS . Obesity and insulin resistance. J Clin Invest 2000; 106: 473–481.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
Roberts MD, Bayless DS, Company JM, Jenkins NT, Padilla J, Childs TE et al. Elevated skeletal muscle irisin precursor FNDC5 mRNA in obese OLETF rats. Metabolism 2013; 62: 1052–1056.
Swick AG, Orena S, O'Connor A . Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass. Metabolism 2013; 62: 1070–1073.
Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract 2013; 100: 96–101.
DeFronzo RA, Ferrannini E, Simonson DC . Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989; 38: 387–395.
Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A et al. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 2000; 49: 1367–1373.
Gerich JE, Nurjhan N . Gluconeogenesis in type 2 diabetes. Adv Exp Med Biol 1993; 334: 253–258.
Perriello G, Pampanelli S, Del Sindaco P, Lalli C, Ciofetta M, Volpi E et al. Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 1997; 46: 1010–1016.
Yki-Jarvinen H . Glucose toxicity. Endocr Rev 1992; 13: 415–431.
Saltiel AR, Kahn CR . Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414: 799–806.
Shih CC, Lin CH, Lin WL, Wu JB . Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol 2009; 123: 82–90.
Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 2014; 99: E2154–E2161.
Pilkis SJ, Granner DK . Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 1992; 54: 885–909.
Goto M, Yoshioka T, Battelino T, Ravindranath T, Zeller WP . TNFalpha decreases gluconeogenesis in hepatocytes isolated from 10-day-old rats. Pediatr Res 2001; 49: 552–557.
He J, Watkins S, Kelley DE . Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 2001; 50: 817–823.
Simoneau JA, Kelley DE . Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol (1985) 1997; 83: 166–171.
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ . Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001; 291: 2613–2616.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79–83.
Hardie DG, Carling D, Carlson M . The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 1998; 67: 821–855.
Winder WW, Hardie DG . AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 1999; 277: E1–E10.
Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014; 63: 514–525.
Wang D, Luo P, Wang Y, Li W, Wang C, Sun D et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes 2013; 62: 1697–1708.
Li H, Song Y, Zhang LJ, Gu Y, Li FF, Pan SY et al. LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid beta-oxidation of lipid droplets. PLoS One 2012; 7: e36712.
Dombrowski L, Roy D, Marcotte B, Marette A . A new procedure for the isolation of plasma membranes, T tubules, and internal membranes from skeletal muscle. Am J Physiol 1996; 270: E667–E676.
Ishibashi J, Seale P . Medicine. Beige can be slimming. Science 2010; 328: 1113–1114.
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J . Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285: 7153–7164.
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61: 1725–1738.
Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013; 98: E769–E778.
Barzilai N, Rossetti L . Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem 1993; 268: 25019–25025.
Foster JD, Pederson BA, Nordlie RC . Glucose-6-phosphatase structure, regulation, and function: an update. Proc Soc Exp Biol Med 1997; 215: 314–332.
Massillon D, Barzilai N, Chen W, Hu M, Rossetti L . Glucose regulates in vivo glucose-6-phosphatase gene expression in the liver of diabetic rats. J Biol Chem 1996; 271: 9871–9874.
Rosella G, Zajac JD, Baker L, Kaczmarczyk SJ, Andrikopoulos S, Adams TE et al. Impaired glucose tolerance and increased weight gain in transgenic rats overexpressing a non-insulin-responsive phosphoenolpyruvate carboxykinase gene. Mol Endocrinol 1995; 9: 1396–1404.
Park JH, Kaushansky K, Levitt L . Transcriptional regulation of interleukin 3 (IL3) in primary human T lymphocytes. Role of AP-1- and octamer-binding proteins in control of IL3 gene expression. J Biol Chem 1993; 268: 6299–6308.
Valera A, Pujol A, Pelegrin M, Bosch F . Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA 1994; 91: 9151–9154.
Carling D . The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci 2004; 29: 18–24.
Kim EJ, Jung SN, Son KH, Kim SR, Ha TY, Park MG et al. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein kinase. Mol Pharmacol 2007; 72: 62–72.
Zhang BB, Zhou G, Li C . AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 2009; 9: 407–416.
Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD et al. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes 2003; 52: 1066–1072.
Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 2005; 54: 1331–1339.
Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C . 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 2000; 49: 896–903.
Merrill GF, Kurth EJ, Hardie DG, Winder WW . AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 1997; 273: E1107–E1112.
Woods A, Salt I, Scott J, Hardie DG, Carling D . The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett 1996; 397: 347–351.
Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mouse. Diabetes 2014; 64: 49–59.
Acknowledgements
This work was supported by Program for National Science Fund for Distinguished Young Scholars of China (Grant No. 81225001), National Key Basic Research Program of China (973 Program, Grant No. 2013CB531204), New Century Excellent Talents in University (Grant No. NCET-11-0870), Program for Changjiang Scholars and Innovative Research Team in University (Grant No. PCSIRT1053), Key Science and Technology Innovation Team in Shaanxi Province (Grant No. 2014KCT-19), National Science Funds of China (Grants No. 81170186, 81470478 and 81400201).
Author contributions
The work presented here was carried out in collaboration between all authors. LT and YL defined the research theme and revised the manuscript critically. CX and JL designed methods and experiments, carried out the laboratory experiments, and wrote the paper. DZ, HW, LX, YL, JY, KL, CX, LZ and QW collected and analyzed the data, and interpreted the results.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xin, C., Liu, J., Zhang, J. et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes 40, 443–451 (2016). https://doi.org/10.1038/ijo.2015.199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.199
This article is cited by
-
Treadmill exercise with nanoselenium supplementation affects the expression of Irisin/FNDC5 and semaphorin 3A in rats exposed to cigarette smoke extract
3 Biotech (2024)
-
The Association of Serum Irisin with Impaired Glucose Before and After Laparoscopic Sleeve Gastrectomy in Obesity
Obesity Surgery (2023)
-
The relationship between sarcopenia detected in newly diagnosed colorectal cancer patients and FGF21, irisin and CRP levels
European Geriatric Medicine (2022)
-
Irisin improves adiposity and exercise tolerance in a rat model of postmenopausal obesity through enhancing adipo-myocyte thermogenesis
Journal of Physiology and Biochemistry (2022)
-
Inverse correlation between serum irisin and cardiovascular risk factors among Chinese overweight/obese population
BMC Cardiovascular Disorders (2021)