Abstract
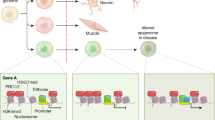
The availability to the DNA strand and the activity of the transcription machinery is crucial for the cell to use the information in the DNA. The epigenetic mechanisms DNA methylation, modification of histone tails, other chromatin-modifying processes and interference by small RNAs regulate the cell-type-specific DNA expression. Epigenetic marks can be more or less plastic perpetuating responses to various molecular signals and environmental stimuli, but in addition apparently stochastic epigenetic marks have been found. There is substantial evidence from animal and man demonstrating that both transient and more long-term epigenetic mechanisms have a role in the regulation of the molecular events governing adipogenesis and glucose homeostasis. Intrauterine exposure such as poor maternal nutrition has consistently been demonstrated to contribute to a particular epigenotype and thereby developmental metabolic priming of the exposed offspring in animal and man. Epigenetic modifications can be passed not only from one cell generation to the next, but metabolic disease-related epigenotypes have been proposed to also be transmitted germ-line. Future more comprehensive knowledge on epigenetic regulation will complement genome sequence data for the understanding of the complex etiology of obesity and related disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hebebrand J, Volckmar AL, Knoll N, Hinney A . Chipping away the ‘missing heritability’: GIANT steps forward in the molecular elucidation of obesity—but still lots to go. Obes Facts 2010; 3: 294–303.
Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 2010; 11: 446–450.
Bird A . Perceptions of epigenetics. Nature 2007; 447: 396–398.
McGowan PO, Szyf M . The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol Dis 2010; 39: 66–72.
Anway MD, Cupp AS, Uzumcu M, Skinner MK . Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005; 308: 1466–1469.
Reik W, Dean W, Walter J . Epigenetic reprogramming in mammalian development. Science 2001; 293: 1089–1093.
Gluckman PD, Hanson MA, Beedle AS . Non-genomic transgenerational inheritance of disease risk. Bioessays 2007; 29: 145–154.
Feinberg AP . Epigenomics reveals a functional genome anatomy and a new approach to common disease. Nat Biotechnol 2010; 28: 1049–1052.
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12: 342–348.
Dhasarathy A, Wade PA . The MBD protein family-reading an epigenetic mark? Mutat Res 2008; 647: 39–43.
Okano M, Bell DW, Haber DA, Li E . DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999; 99: 247–257.
Leonhardt H, Page AW, Weier HU, Bestor TH . A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 1992; 71: 865–873.
Illingworth RS, Bird AP . CpG islands–‘a rough guide’. FEBS Lett 2009; 583: 1713–1720.
Shiota K, Kogo Y, Ohgane J, Imamura T, Urano A, Nishino K et al. Epigenetic marks by DNA methylation specific to stem, germ and somatic cells in mice. Genes Cells 2002; 7: 961–969.
Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet 2006; 38: 1378–1385.
Illingworth R, Kerr A, Desousa D, Jørgensen H, Ellis P, Stalker J et al. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol 2008; 6: e22.
Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet 2009; 41: 178–186.
Feber A, Wilson GA, Zhang L, Presneau N, Idowu B, Down TA et al. Comparative methylome analysis of benign and malignant peripheral nerve sheath tumors. Genome Res 2011; 21: 515–524.
Marti A, Ordovas J . Epigenetics lights up the obesity field. Obes Facts 2011; 4: 187–190.
Wu SC, Zhang Y . Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 2010; 11: 607–620.
Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–10609.
Poulsen P, Esteller M, Vaag A, Fraga MF . The epigenetic basis of twin discordance in age-related diseases. Pediatr Res 2007; 61: 38R–42R.
Kriaucionis S, Heintz N . The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009; 324: 929–930.
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324: 930–935.
Jin SG, Wu X, Li AX, Pfeifer GP . Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res 2011; 39: 5015–5024.
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y . Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466: 1129–1133.
Rauch TA, Pfeifer GP . DNA methylation profiling using the methylated-CpG island recovery assay (MIRA). Methods 2010; 52: 213–217.
Kouzarides T . Chromatin modifications and their function. Cell 2007; 128: 693–705.
Haberland M, Montgomery RL, Olson EN . The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 2009; 10: 32–42.
Zhou VW, Goren A, Bernstein BE . Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet 2011; 12: 7–18.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A . An operational definition of epigenetics. Genes Dev 2009; 23: 781–783.
Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233.
Barski A, Jothi R, Cuddapah S, Cui K, Roh TY, Schones DE et al. Chromatin poises miRNA- and protein-coding genes for expression. Genome Res 2009; 19: 1742–1751.
Feinberg AP, Vogelstein B . Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983; 301: 89–92.
Frigola J, Song J, Stirzaker C, Hinshelwood RA, Peinado MA, Clark SJ . Epigenetic remodeling in colorectal cancer results in coordinate gene suppression across an entire chromosome band. Nat Genet 2006; 38: 540–549.
Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 2005; 37: 391–400.
Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet 2009; 41: 1350–1353.
Feinberg AP, Irizarry RA . Evolution in health and medicine Sackler colloquium: stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc Natl Acad Sci USA 2010; 107 (Suppl 1): 1757–1764.
Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F et al. Insulin gene expression is regulated by DNA methylation. PLoS One 2009; 4: e6953.
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009; 49: 868–913.
Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA . Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 2009; 139: 1054–1060.
Widiker S, Karst S, Wagener A, Brockmann GA . High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet 2010; 51: 193–197.
Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol 2009; 587: 4963–4976.
Yokomori N, Tawata M, Onaya T . DNA demethylation during the differentiation of 3T3-L1 cells affects the expression of the mouse. GLUT4 gene. Diabetes 1999; 48: 685–690.
Fujiki K, Kano F, Shiota K, Murata M . Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol 2009; 7: 38.
Okamura M, Inagaki T, Tanaka T, Sakai J . Role of histone methylation and demethylation in adipogenesis and obesity. Organogenesis 2010; 6: 24–32.
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007; 318: 1469–1472.
Tateishi K, Okada Y, Kallin EM, Zhang Y . Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 2009; 458: 757–761.
Stepanow S, Reichwald K, Huse K, Gausmann U, Nebel A, Rosenstiel P et al. Allele-specific, age-dependent and BMI-associated DNA methylation of human MCHR1. PLoS One 2011; 6: e17711.
Campión J, Milagro F, Martínez JA . Epigenetics and obesity. Prog Mol Biol Transl Sci 2010; 94: 291–347.
Barrès R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 2009; 10: 189–198.
Heneghan HM, Miller N, Kerin MJ . Role of microRNAs in obesity and the metabolic syndrome. Obes Rev 2010; 11: 354–361.
Bengestrate L, Virtue S, Campbell M, Vidal-Puig A, Hadaschik D, Hahn P et al. Genome-wide profiling of microRNAs in adipose mesenchymal stem cell differentiation and mouse models of obesity. PLoS One 2011; 6: e21305.
Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One 2010; 5: e9022.
Jirtle RL, Skinner MK . Environmental epigenomics and disease susceptibility. Nat Rev Genet 2007; 8: 253–262.
Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J 2010; 24: 3135–3144.
Feinberg AP, Irizarry RA, Fradin D, Aryee MJ, Murakami P, Aspelund T et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med 2010; 2: 49ra67.
O’Hara A, Lim FL, Mazzatti DJ, Trayhurn P . Microarray analysis identifies matrix metalloproteinases (MMPs) as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium. Pflugers Arch 2009; 458: 1103–1114.
Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: Role of oxidative stress. Circ Res 2001; 88: 1291–1298.
Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 2003; 278: 11888–11896.
Kaun KR, Sokolowski MB . cGMP-dependent protein kinase: linking foraging to energy homeostasis. Genome 2009; 52: 1–7.
Goldstone AP . Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab 2004; 15: 12–20.
Le Stunff C, Fallin D, Bougneres P . Paternal transmission of the very common class I INS VNTR alleles predisposes to childhood obesity. Nat Genet 2001; 29: 96–99.
Gluckman PD, Hanson MA . Developmental plasticity and human disease: research directions. J Intern Med 2007; 261: 461–471.
Bruce KD, Cagampang FR . Epigenetic priming of the metabolic syndrome. Toxicol Mech Methods 2011; 21: 353–361.
Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2–18 years. Pediatrics 2006; 118: e1644–e1649.
Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ . Chronic high-fat diet in fathers programs s-cell dysfunction in female rat offspring. Nature 2010; 467: 963–966.
Dumesic DA, Patankar MS, Barnett DK, Lesnick TG, Hutcherson BA, Abbott DH . Early prenatal androgenization results in diminished ovarian reserve in adult female rhesus monkeys. Hum Reprod 2009; 24: 3188–3195.
Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C . Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology 2010; 151: 595–605.
Dunn GA, Bale TL . Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 2009; 150: 4999–5009.
Morgan HD, Sutherland HG, Martin DI, Whitelaw E . Epigenetic inheritance at the agouti locus in the mouse [see comments]. Nat Genet 1999; 23: 314–318.
Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA . Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 2007; 97: 435–439.
Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC . Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR-alpha promoter of the offspring. Br J Nutr 2008; 100: 278–282.
Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC . Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr 2007; 97: 1064–1073.
van Straten EM, Bloks VW, Huijkman NC, Baller JF, van Meer H, Lütjohann D et al. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol 2010; 298: R275–R282.
Kim YI . Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr 2005; 135: 2703–2709.
Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS et al. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA 2007; 104: 12796–12800.
Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J et al. Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS One 2010; 5: e14398.
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM . Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010; 151: 4756–4764.
Rubin BS, Murray MK, Damassa DA, King JC, Soto AM . Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 2001; 109: 675–680.
Dolinoy DC, Huang D, Jirtle RL . Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 2007; 104: 13056–13061.
Mehedint MG, Craciunescu CN, Zeisel SH . Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci USA 2010; 107: 12834–12839.
Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008; 105: 17046–17049.
Lumey LH, Stein AD, Kahn HS, Romijn JA . Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr 2009; 89: 1737–1743.
Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes 2011; 60: 1528–1534.
Ling C, Groop L . Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 2009; 58: 2718–2725.
Pirola L, Balcerczyk A, Okabe J, El-Osta A . Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol 2010; 6: 665–675.
Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008; 41: 91–102.
Staels B . When the clock stops ticking, metabolic syndrome explodes. Nat Med 2006; 12: 54–55.
Takahashi JS, Hong HK, Ko CH, McDearmon EL . The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet 2008; 9: 764–775.
Ripperger JA, Merrow M . Perfect timing: epigenetic regulation of the circadian clock. FEBS Lett 2011; 585: 1406–1411.
Brown SE, Fraga MF, Weaver IC, Berdasco M, Szyf M . Variations in DNA methylation patterns during the cell cycle of HeLa cells. Epigenetics 2007; 2: 54–65.
Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U . Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 2004; 119: 693–705.
Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A et al. Regulation of clock-controlled genes in mammals. PLoS One 2009; 4: e4882.
la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM . A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 2001; 50: 1237–1243.
Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004; 2: e377.
Green CB, Takahashi JS, Bass J . The meter of metabolism. Cell 2008; 134: 728–742.
Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R et al. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J 2011; 25: 714–726.
Benyshek DC, Johnston CS, Martin JF . Glucose metabolism is altered in the adequately-nourished grand-offspring (F3 generation) of rats malnourished during gestation and perinatal life. Diabetologia 2006; 49: 1117–1119.
Dunn GA, Bale TL . Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011; 152: 2228–2236.
Kaati G, Bygren LO, Edvinsson S . Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet 2002; 10: 682–688.
Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006; 14: 159–166.
Richards EJ . Inherited epigenetic variation–revisiting soft inheritance. Nat Rev Genet 2006; 7: 395–401.
Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ . Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann NY Acad Sci 2004; 1024: 182–212.
Cropley JE, Suter CM, Beckman KB, Martin DI . Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci USA 2006; 103: 17308–17312.
Feinberg AP . Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447: 433–440.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lavebratt, C., Almgren, M. & Ekström, T. Epigenetic regulation in obesity. Int J Obes 36, 757–765 (2012). https://doi.org/10.1038/ijo.2011.178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.178
Keywords
This article is cited by
-
Investigation the interaction of dietary fat quality indices and the MC4R gene in metabolically healthy and unhealthy overweight and obese women
Scientific Reports (2023)
-
Genome-wide screening for genetic variants in polyadenylation signal (PAS) sites in mouse selection lines for fatness and leanness
Mammalian Genome (2023)
-
Variably methylated retrotransposons are refractory to a range of environmental perturbations
Nature Genetics (2021)
-
Comparison of visceral adipose tissue DNA methylation and gene expression profiles in female adolescents with obesity
Diabetology & Metabolic Syndrome (2019)
-
Identification and characterization of long non-coding RNAs in subcutaneous adipose tissue from castrated and intact full-sib pair Huainan male pigs
BMC Genomics (2017)