Abstract
Introduction:
Oxyntomodulin (OXM) is a gut hormone released from intestinal L cell. Synthetic OXM and its analog reduce food intake and body weight in both rodents and human beings by being administered intravenously. However, people find intravenous administration difficult because of its side effects and inconvenience. The aim of this study is to develop a novel oral delivery system for OXM and its analog using genetically engineered Bifidobacterium as the carrier.
Methods:
An OXM gene expression vector pBBADs-OXM for the Bifidobacterium genus was constructed. Human OXM sequence was fused with extracellular exo-xylanase (XynF) signal peptide (Xs) from Bifidobacterium longum under the control of the pBAD promoter. B. longum NCC2705 was transformed with the recombinant plasmid pBBADs-OXM by electroporation, and the transformed B. longum was selected using MRS plates containing 60 μg ml−1 ampicillin. The OXM expression in vitro was identified by western blot and enzyme-linked immunosorbent assay (ELISA) assay after L-arabinose induction. Overweight BALB/c mice were treated with B. longum transformed with OXM after 0.2% L-arabinose induction every day for 4 weeks to investigate the effects of OXM-transformed B. longum on food intake and body weight by oral administration. The B. longum transformed with the green fluorescent protein (GFP) gene was used as negative control; orlistat, a gastrointestinal lipase inhibitor, was used as positive control; Normal saline (NS, 0.9% saline) was used as blank control. The food intakes of each group were measured every day, and body weights were measured once a week. Normal BALB/c (2 months old) mice were treated with OXM-transformed B. longum after induction by intragastric administration every day for 6 days to reveal the mechanism of transformed B. longum, with OXM exerting its biological function by oral administration. Plasma OXM, plasma ghrelin and the OXM of intestinal contents were detected by the ELISA method. Plasma glucose and triglyceride levels were analyzed using the Automatic Biochemistry Analyzer.
Results:
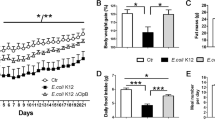
Transformed B. longum with OXM was selected and identified without biological and morphological alteration. An approximately 4–5 kDa OXM peptide was detected in both the supernatant and the cell pellet of transformed B. longum after L-arabinose induction in vitro. The food intake, body weight and blood triglyceride level of overweight mice treated with OXM-transformed B. longum were all significantly reduced compared with that of the GFP negative control group and NS control group (P<0.01). Interestingly, the plasma triglyceride level of the GFP group was significantly decreased compared with that of the NS control group (P<0.01). The OXM level in the intestinal contents of the OXM group was significantly increased compared with that of the GFP negative control group and the NS group (P<0.05). The plasma ghrelin level of the OXM group was significantly decreased compared with that of the GFP and NS groups (P<0.01). Unexpectedly, the ghrelin level of the GFP group was significantly increased compared with that of the NS control group (P<0.01).
Conclusion:
A novel oral delivery system of Bifidobacterium for human OXM has been successfully established. The expression of recombinant OXM can be detected in the supernatant and cell pellet of transformed B. longum. OXM-transformed B. longum reduces food intake, body weight and plasma lipid level in overweight mice by oral administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Bataille D, Tatemoto K, Gespach C, Jornvall H, Rosselin G, Mutt V . Isolation of glucagon-37 (bioactive enteroglucagon/oxyntomodulin) from porcine jejuno-ileum. Characterization of the peptide. FEBS Lett 1982; 146: 79–86.
Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF . Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986; 261: 11880–11889.
Lui EY, Asa SL, Drucker DJ, Lee YC, Brubaker PL . Glucagon and related peptides in fetal rat hypothalamus in vivo and in vitro. Endocrinology 1990; 126: 110–117.
Dubrasquet M, Bataille D, Gespach C . Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci Rep 1982; 2: 391–395.
Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ . Oxyntomodulin from distal gut. Role in regulation of gastric and pancreatic functions. Dig Dis Sci 1989; 34: 1411–1419.
Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR . Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 1983; 57: 488–495.
Besterman HS, Cook GC, Sarson DL, Christofides ND, Bryant MG, Gregor M et al. Gut hormones in tropical malabsorption. Br Med J 1979; 2: 1252–1255.
Sarson DL, Scopinaro N, Bloom SR . Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes 1981; 5: 471–480.
Holst JJ, Sorensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB et al. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol 1979; 14: 205–207.
Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology 2001; 142: 4244–4250.
Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 2004; 145: 2687–2695.
Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005; 54: 2390–2395.
Baggio LL, Huang Q, Brown TJ, Drucker DJ . Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004; 127: 546–558.
Wynne K, Bloom S . Oxyntomodulin: a new therapy for obesity? Sci Sch Autumn 2007; 6: 25–29.
De Simone C, Rosati E, Moretti S, Salvadori B, Vesely R, Jirillo E . Probiotics and stimulation of the immune response. Eur J Clin Nutr 1991; 45: 32–34.
Elmer GW, Surawicz CM, McFarl LV . Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA 1996; 275: 870–8766.
Orrhage K, Sillerstrom E, Gustafsson JA, Nord CE, Rafter J . Binding of mutagenic heterocyclic amines by intestinal and lactic acid bacteria. Mutat Res 1994; 311: 239–248.
Singh J, Rivenson A, Tomita M, Shimamura S, Ishibashi N, Reddy BS . Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis 1997; 18: 833–841.
Nakamura T, Sasaki T, Fujimori M . Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem 2002; 66: 2362–2366.
Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang JJ et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther 2003; 10: 105–111.
Escogido MLR, Rodríguez ADL, de la Rosa APB . A novel binary expression vector for production of human IL-10 in Escherichia coli and Bifidobacterium longum. Biotechnol Lett 2007; 29: 1249–1253.
Moon GS, Pyun YR, Park MS, Ji GE, Kim WJ . Secretion of recombinant pediocin PA-1 by Bifidobacterium longum, Using the signal sequence for bifidobacterial α-amylase. Appl Environ Microbiol 2005; 72: 5630–5632.
Zhu ZS, Wang LS, Zeng WS, Zheng YJ, Luo SQ, Li YX et al. Isolation and identification of a Bifidobacterium strain with a plasmid polymerase by PCR. J Microecol (China) 2007; 19: 321–323.
Argnani A, Leer RJ, van Luijk N, Pouwels PH . A convenient and reproducible method to genetically transform bacteria of the genus Bifidobacterium. Microbiology 1996; 142: 109–114.
De Roos NM, Katan MB . Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am J Clin Nutr 2000; 71: 405–411.
Xiao JZ, Kondo S, Takahashi N, Miyaji K, Oshida K, Hiramatsu A et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci 2003; 86: 2452–2461.
Ataie-Jafari A, Larijani B, Majd HA, Tahbaz F . Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann Nutr Metab 2009; 54: 22–27.
Castell JV, Friedrich G, Kuhn CS, Poppe GE . Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. Gastrointest Liver Physiol 1997; 273: 139–146.
Worthington BS, Boatman ES, Kenny GE . Intestinal absorption of intact proteins in normal and protein-deficient rats. Am J Clin Nutr 1974; 27: 276–286.
Gros L, Thorens B, Bataille D, Kervran A . Glucagon-like peptide-1-(7–36) amide, oxyntomodulin, and glucagon interact with a common receptor in a somatostatin-secreting cell line. Endocrinology 1993; 133: 631–638.
Schepp W, Dehne K, Riedel T, Schmidtler J, Schaffer K, Classen M . Oxyntomodulin: a cAMP-dependent stimulus of rat parietal cell function via the receptor for glucagon-like peptide-1 (7–36) NH2. Digestion 1996; 57: 398–405.
Wei Y, Mojsov S . Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 1995; 358: 219–224.
Bullock B, Heller R, Habener J . Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996; 137: 2968–2978.
Lin CW, Miller TR . Both CCK-A and CCK-B/gastrin receptors are present on rabbit vagus nerve. Am J Physiol Regul Integr Comp Physiol 1992; 263: 591–595.
Corp ES, Smith GP . Characterization of axonally transported [125I]PYY binding sites in rat vagus nerve. Brain Res 1991; 553: 175–179.
Anini Y, Jarrousse C, Chariot J, Nagain C, Yanaihara N, Sasaki K et al. xyntomodulin inhibits pancreatic secretion through the nervous system in rats. Pancreas 2000; 20: 348–360.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS . A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719.
Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D . Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 2004; 287: E297–E304.
Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR . Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats. Am J Physiol Endocrinol Metab 2002; 283: E1173–E1177.
Patterson M, Murphy KG, Patel SR, Patel NA, Greenwood HC, Cooke JH et al. Hypothalamic injection of oxyntomodulin suppresses circulating ghrelin-like immunoreactivity. Endocrinology 2009; 150: 3513–3520.
Acknowledgements
We express our thanks to the Clinical Ecsomatic laboratory of the Southern Hospital for their invaluable contribution to the detection of some blood biochemical parameters. This research was funded by the National Natural Science Foundation of China, (NSFC:30472001), by the Science and Technology Planning Project of Guangdong Province (2006B3552005) by and the Technology Planning Project of Zhongshan city, Guangdong Province (2006A119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Long, R., Zeng, W., Chen, L. et al. Bifidobacterium as an oral delivery carrier of oxyntomodulin for obesity therapy: inhibitory effects on food intake and body weight in overweight mice. Int J Obes 34, 712–719 (2010). https://doi.org/10.1038/ijo.2009.277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.277
Keywords
This article is cited by
-
Engineered probiotics introduced to improve intestinal microecology for the treatment of chronic diseases: present state and perspectives
Journal of Diabetes & Metabolic Disorders (2023)
-
Oral immunization of mice using Bifidobacterium longum expressing VP1 protein from enterovirus 71
Archives of Virology (2013)
-
Improved adhesive properties of recombinant bifidobacteria expressing the Bifidobacterium bifidum-specific lipoprotein BopA
Microbial Cell Factories (2012)