Abstract
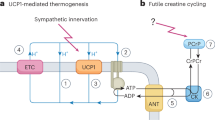
Obesity is endemic in many regions of the world and a forerunner of several serious and sometimes fatal diseases such as ischemic heart disease, stroke, kidney failure and neoplasia. Although we know its origin—it results when energy intake exceeds energy expenditure—at present, the only proven therapy is bariatric surgery. This is a major abdominal procedure that, for reasons that are largely unknown (it cannot be explained solely by a reduction in ventricular volume), significantly reduces energy intake, but because of cost and limited availability, it will most likely be reserved for only a small fraction of those who stand to gain from effective antiobesity treatment. Clearly, alternative ways to treat obesity are needed. Another way to combat excessive accumulation of white adipose tissue would be to increase energy expenditure. Rodents, hibernators and human infants all have a specialized tissue—brown adipose tissue (BAT)—with the unique capacity to regulate energy expenditure by a process called adaptive thermogenesis. This process depends on the expression of uncoupling protein-1 (UCP1), which is a unique marker for BAT. UCP1 is an inner mitochondrial membrane protein that short circuits the mitochondrial proton gradient, so that oxygen consumption is no longer coupled to adenosine triphosphate synthesis. As a consequence, heat is generated. Mice lacking ucp-1 are severely compromised in their ability to maintain normal body temperature when acutely exposed to cold and they are also prone to become obese. We have shown that, in mice, BAT protects against diet-induced obesity, insulin resistance and type 2 diabetes. This is based on prevention of excessive accumulation of triglyceride in non-adipose tissues such as muscle and liver. Ectopic triglyceride storage at these locations is associated with initiation of insulin resistance and, ultimately, development of type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Cypress AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND et al. Cold-activated adipose tissue in healthy adult men. N Engl J Med 2009; 360: 1500–1508.
Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357: 753–761.
Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–752.
Lowell BB, Spiegelman BM . Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404: 652–660.
Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997; 387: 90–94.
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J . UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203–209.
Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S . FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001; 106: 563–573.
Kim JK, Kim HJ, Park SY, Cederberg A, Westergren R, Nilsson D et al. Adipocyte-specific overexpression of FOXC2 prevents diet-induced increases in intramuscular fatty acyl CoA and insulin resistance. Diabetes 2005; 54: 1657–1663.
Unger RH, Orci L . Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord 2000; 24 (Suppl 4): S28–S32.
Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006; 296: 164–176.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 2007; 104: 4401–4406.
Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE et al. White fat progenitor cells reside in the adipose vasculature. Science 2008; 322: 583–586.
Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP . Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007; 48: 41–51.
Civitarese AE, Ravussin E . Mitochondrial energetics and insulin resistance. Endocrinology 2008; 149: 950–954.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P . Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005; 434: 113–118.
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267–273.
Bjorntorp P, Schersten T, Fagerberg SE . Respiration and phosphorylation of mitochondria isolated from the skeletal muscle of diabetic and normal subjects. Diabetologia 1967; 3: 346–352.
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008; 7: 45–56.
Asin-Cayuela J, Gustafsson CM . Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem Sci 2007; 32: 111–117.
Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G . Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 2009; 9: 265–276.
Ma SW, Foster DO . Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol 1986; 64: 609–614.
Iyer RB, Guo CC, Perrier N . Adrenal pheochromocytoma with surrounding brown fat stimulation. AJR Am J Roentgenol 2009; 192: 300–301.
Ribeiro MO, Bianco SD, Kaneshige M, Schultz JJ, Cheng SY, Bianco AC et al. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-{beta} isoform specific and required for adaptive thermogenesis. Endocrinology 2010; 151: 432–440.
Acknowledgements
I apologize to all colleagues who have been cited only cursorily, or have not been cited because of space constraints. This work was supported by generous grants from the Söderberg Foundation, the Swedish Research Council (Grant K2005-32BI-15324-01A), the Arne and IngaBritt Lundberg Foundation, the Knut and Alice Wallenberg Foundation and the Swedish Foundation for Strategic Research through the Center for Cardiovascular and Metabolic Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Enerbäck, S. Brown adipose tissue in humans. Int J Obes 34 (Suppl 1), S43–S46 (2010). https://doi.org/10.1038/ijo.2010.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.183
Keywords
This article is cited by
-
Combined training increases thermogenic fat activity in patients with overweight and type 2 diabetes
International Journal of Obesity (2022)
-
Role of edible mushroom as a potent therapeutics for the diabetes and obesity
3 Biotech (2019)
-
Observed changes in brown, white, hepatic and pancreatic fat after bariatric surgery: Evaluation with MRI
European Radiology (2019)
-
Pleomorphic liposarcoma of bone: a rare primary malignant bone tumour
Clinical Sarcoma Research (2018)
-
Anti-obesogenic and antidiabetic effects of plants and mushrooms
Nature Reviews Endocrinology (2017)