Abstract
Objective:
Several studies demonstrated that obese subjects have a hyperactive hypothalamic-pituitary-adrenal axis and that sex steroid hormones have been closely related to the regulation of adiposity, either through direct or indirect physiological mechanisms. Allopregnanolone (3α-hydroxy-5α-pregn-20-one; AP) is a circulating neuroactive steroid hormone involved in the modulation of behavioral functions, stress and neuroendocrine axis. The aim of our study was to evaluate basal serum AP levels in obese children.
Subjects and measurements:
We studied 27 normal weight (NW) and 23 overweight (OW) girls. Gonadotropins and steroid hormones were assessed in all patients.
Results:
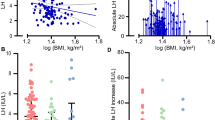
Basal AP concentrations in OW girls were significantly higher than in NW controls (P=0.013). There was no difference found between the other gonadal and adrenal hormones. Considering the pubertal stage, we demonstrated that obese pubertal girls presented higher AP concentrations than prepubertal and pubertal NW ones (P=0.020), and higher dehydroepiandrosterone sulfate (DHEAS) levels with respect to prepubertal obese girls, and prepubertal and pubertal NW patients (P=0.025). AP and DHEAS were significantly directly related to weight (r=0.31 and r=0.54, respectively) and body mass index (r=0.29 and r=0.34, respectively). In pubertal OW girls, a significant positive correlation between AP and DHEAS (r=0.60), A (r=0.72) and luteinizing hormone (r=0.64) levels was demonstrated.
Conclusion:
The present study demonstrates that AP is hypersecreted in children and adolescent with OW involving DHEAS concentrations, too. Our data suggest a possible role of AP in the regulation of neuroendocrine axis related to obesity. We can also speculate that in OW girls, who could manifest emotional and behavioral problems, a part of higher levels of this neuroactive steroid might act as γ-aminobutyric acid agonist producing anxiolytic-sedative effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Livingstone DEW, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ et al. Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology 2000; 141: 560–563.
Genazzani AR, Pintor C, Corda R . Plasma levels of gonadotropins, prolactin, thyroxin, adrenal and gonadal steroids in obese prepubertal girls. J Clin Endocrinol Metab 1978; 47: 974–979.
Pintor C, Loche S, Faedda A, Fanni V, Nurchi AM, Corda R . Adrenal androgens in obese boys before and after weight loss. Horm Metab Res 1984; 16: 544–548.
Ibanez L, Potau N, Marcos MV, de Zegher F . Corticotropin-releasing hormone: a potent androgen secretagogue in girls with hyperandrogenism after precocious pubarche. J Clin Endocrinol Metab 1999; 84: 4602–4606.
Oppenheimer E, Linder B, DiMartino-Nardi J . Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab 1995; 80: 614–618.
Maccario M, Mazza E, Ramunni J, Oleandri SE, Savio P, Grottoli S et al. Relationship between dehydroepiandrosterone-sulphate and anthropometric, metabolic and hormonal variables in a large cohort of obese women. Clin Endocrinol 1999; 50: 595–600.
l’Allemand D, Schmidt S, Rousson V, Brabant G, Gasse T, Gruters A . Association between body mass, leptin, IGF-1 and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol 2002; 146: 537–543.
Baulieu EE . Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 1998; 23: 963–987.
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM . Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986; 232: 1004–1007.
Wetzel CH, Hermann B, Behl C, Pestel E, Rammes G, Zieglgansberger W et al. Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor. Mol Endocrinol 1998; 12: 1441–1451.
Rupprecht R . Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003; 28: 139–168.
Dubrovsky BO . Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 169–192.
Patchev VK, Shoaib M, Holsboer F, Almeida OFX . The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994; 62: 265–271.
Patchev VK, Moritkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OFX . Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest 1997; 99: 962–966.
Pham J, Porter J, Svec D, Eiswirth C, Svec F . The effect of dehydroepiandrosterone on Zucker rats selected for fat food preference. Physiol Behav 2000; 70: 431–441.
Reddy DS, Kulkarni SK . The role of GABA-A and mitochondrial diazepam-binding inhibitor receptors on the effects of neurosteroids on food intake in mice. Psychopharmacology 1998; 137: 391–400.
Berridge KC, Treit D . Chlordiazepoxide directly enhances positive ingestive reactions in rats. Pharmacol Biochem Behav 1986; 24: 217–221.
Treit D, Berridge KC, Schultz CE . The direct enhancement of positive palatability by chlordiazepoxide is antagonized by Ro 15-1788 and CGS 8216. Pharmacol Biochem Behav 1987; 26: 709–714.
Parker LA . Chlordiazepoxide nonspecifically enhances consumption of saccharin solution. Pharmacol Biochem Behav 1991; 38: 375–377.
Parker LA . Chlordiazepoxide enhances the palatability of lithium-, amphetamine-, and saline-paired saccharin solution. Pharmacol Biochem Behav 1995; 50: 345–349.
Higgs S, Cooper SJ . Antineophobic effect of the neuroactive steroid 3α-hydroxy-5β-pregnan-20-one in males rats. Pharmacol Biochem Behav 1988; 60: 125–131.
Monteleone P, Luisi M, Colurcio B, Casarosa E, Monteleone P, Ioime R et al. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med 2001; 63: 62–68.
Monteleone P, Luisi M, de Filippis G, Colurcio B, Monteleone P, Genazzani AR et al. Circulating levels of neuroactive steroids in patients with binge eating disorder: a comparison with nonobese healthy controls and non-binge eating obese subjects. Int J Eat Disord 2003; 34: 432–440.
Fadalti M, Petraglia F, Luisi S, Bernardi F, Casarosa E, Ferrari E et al. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr Res 1999; 46: 323–327.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243.
Marshall WA, Tanner JM . Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44: 291–303.
Greulich WW, Pyle SI . Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2nd ed. Stanford University Press: Stanford, CA, 1959.
Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A,et al. Circulating levels of allopregnanolone in humans: gender, age and endocrine influences. J Clin Endocrinol Metab 1998; 83: 2099–2103.
Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S et al. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol 1998; 138: 316–321.
Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab 2000; 85: 2429–2433.
Bitran D, Hilvers RJ, Kellogg CK . Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one. Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res 1991; 561: 157–161.
Biggio G, Purdy RH . Neurosteroid and Brain Function. International Review of Neurobiology, vol. 46. Academic Press: San Diego, CA, 2001.
Barbaccia ML, Lello S, Sidiropoulou T, Cocco T, Sorge SP, Cocchiarale A,et al. Plasma 5α-androstane-3α-, 17β-diol, an endogenous steroid that positively modulates GABA receptor function and anxiety: a study in menopausal women. Psychoneuroendocrinology 2000; 25: 659–675.
Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovicg-Djukic S et al. Neurosteroids and panic disorder. Psychiatry Res 2003; 118: 107–116.
Erickson SJ, Robinson TN, Haydel KF, Killen JD . Are overweight children unhappy? Body mass index, depressive symptoms, and overweight concerns in elementary school children. Arch Pediatr Adolesc Med 2000; 154: 931–935.
Sweeting H, Wright C, Minnis H . Psychosocial correlates of adolescent obesity, ‘slimming down’ and ‘becoming obese’. J Adolesc Health 2005; 37: 409e9–409e17.
Anderson SE, Cohen P, Naumova EN, Must A . Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med 2006; 160: 285–291.
Vallée M, George O, Vitiello S, Le Moal M, Mayo W . New insights into the role of neuroactive steroids in cognitive aging. Exp Gerontol 2004; 39: 1695–1704.
Kaur G, Kulkarni SK . Subchronic studies on modulation of feeding behavior and body weight by neurosteroids in female mice. Methods Find Exp Clin Pharmacol 2001; 23: 115–119.
Al-Harithy RN . Dehydroepiandrosterone sulfate levels in women. Relationships with body mass index, insulin and glucose levels. Saudi Med J 2003; 24: 837–841.
Moyer AE, Rodin J, Grilo CM, Cummings N, Larson LM, Rebuffe’-Scrive M . Stress-induced cortisol response and fat distribution in women. Obes Res 1994; 2: 255–261.
Pasquali R, Vicennati V, Calzoni F, Gnudi U, Gambineri A, Ceroni L et al. α2-Adrenoreceptor regulation of the hypothalamic-pituitary-adrenocortical axis in obesity. Clin Endocrinol 2000; 52: 413–421.
Menozzi R, Florio P, Bondi M, Luisi S, Cobellis L, Genazzani AR et al. Increased response of plasma allopregnanolone to corticotropin-releasing hormone in obese patients. Neuroendocrinology 2002; 75: 124–129.
Griffith LD, Mellon SH . Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 1999; 96: 13512–13517.
Mellon SH . Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab 1994; 78: 1003–1008.
Rupprecht R, Hauser CA, Trapp T, Holsboer F . Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol 1996; 56: 163–168.
Fudge MA, Kavaliers M, Ossenkopp K-P . Allopregnanolone produces hyperphagia by reducing neophobia without altering food palatability. Eur Neuropsychopharmacol 2005 (in press; E-pub ahead of print).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Predieri, B., Luisi, S., Casarosa, E. et al. High basal serum allopregnanolone levels in overweight girls. Int J Obes 31, 543–549 (2007). https://doi.org/10.1038/sj.ijo.0803406
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803406