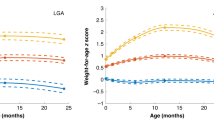
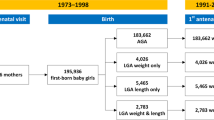
Abstract
Epidemiological studies indicate that children born small for gestational age (SGA) have an increased risk of metabolic and cardiovascular disorders as adults. This suggests that foetal undernutrition leads to permanent metabolic alterations, which predispose to metabolic abnormalities upon exposure to environmental factors such as low physical activity and/or high-energy intake in later life (thrifty phenotype hypothesis). However, this relationship is not restricted to foetal undernutrition or intrauterine growth retardation, but is also found for children born premature, or for high birth weight children. Furthermore, early post-natal nutrition, and more specifically catch-up growth, appear to modulate cardiovascular risk as well. Intrauterine growth retardation can be induced in animal models by energy/protein restriction, or ligation of uterine arteries. In such models, altered glucose homeostasis, including low beta-cell mass, low insulin secretion and insulin resistance is observed after a few weeks of age. In humans, several studies have confirmed that children born SGA have insulin resistance as adolescents and young adults. Alterations of glucose homeostasis and increased lipid oxidation can indeed be observed already in non-diabetic children born SGA at early pubertal stages. These children also have alterations of stature and changes in body composition (increased fat mass), which may contribute to the pathogenesis of insulin resistance. Permanent metabolic changes induced by foetal/early neonatal nutrition (metabolic inprinting) may involve modulation of gene expression through DNA methylation, or alterations of organ structure. It is also possible that events occurring during foetal/neonatal development lead to long-lasting alterations of the hypothalamo-pituitary-adrenal axis or the hypothalamo-pituitary-insulin-like growth factor-1 axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Barker DJ, Osmond C . Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1: 1077–1081.
Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS . Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993; 36: 62–67.
Barker DJ, Eriksson JG, Forsen T, Osmond C . Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235–1239.
Leon DA, Johansson M, Rasmussen F . Gestational age and growth rate of fetal mass are inversely associated with systolic blood pressure in young adults: an epidemiologic study of 165, 136 Swedish men aged 18 years. Am J Epidemiol 2000; 152: 597–604.
Phipps K, Barker DJP, Hales CN, Fall CHD, Osmond C, Clark PMS . Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993; 36: 225–228.
Reaven GM, Lithell H, Landsberg L . Hypertension and associated metabolic abnormalities – the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996; 334: 374–381.
Painter RC, Roseboom TJ, Bleker OP . Prenatal exposure to the Dutch famine and disease in later life: an overview. Reproductive Toxicol 2005; 20: 345–352.
Iglesias-Barreira V, Ahn MT, Reusens B, Dahri S, Hoet JJ, Remacle C . Pre- and postnatal low protein diet affect pancreatic islet blood flow and insulin release in adult rats. Endocrinology 1996; 137: 3797–3801.
Garofano A, Czernichow P, Breant B . Beta-cell mass and proliferation following late fetal and early postnatal malnutrition in the rat. Diabetologia 1998; 41: 114–120.
Simmons RA, Templeton LJ, Gertz SJ . Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001; 50: 2279–2286.
Hales CN, Barker DJ . Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601.
Richardus JH, Graafmans WC, Verloove-Vanhorick SP, Mackenbach JP . Differences in perinatal mortality and suboptimal care between 10 European regions: results of an international audit. Int J Obstetrics Gynaecol 2003; 110: 97–105.
Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS . Premature birth and later insulin resistance. N Engl J Med 2004; 351: 2888.
Hales CN, Ozanne SE . The dangerous road of catch-up growth. J Physiol 2003; 547: 5–10.
Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ . Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 1996; 94: 3246–3250.
Holemans K, Van Bree R, Verhaeghe J, Meurrens K, Van Assche FA . Maternal semistarvation and streptozotocin-diabetes in rats have different effects on the in vivo glucose uptake by peripheral tissues in their female adult offspring. J Nutr 1997; 127: 1371–1376.
Bertin E, Gangnerau MN, Bailbe D, Portha B . Glucose metabolism and beta-cell mass in adult offspring of rats protein and/or energy restricted during the last week of pregnancy. Am J Physiol Endocrinol Metab 1999; 277: E11–E17.
Bertin E, Gangnerau MN, Bellon G, Bailbe D, Arbelot de Vacqueur A, Portha B . Development of beta-cell mass in fetuses of rats deprived of protein and/or energy in last trimester of pregnancy. Am J Physiol Regul Integr Comp Physiol 2002; 283: R623–R630.
De Prins FA, Van Assche FA . Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Biol Neonate 1982; 41: 16–21.
Jansson T, Lambert GW . Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3-4 months of age. J Hypertens 1999; 17: 1239–1248.
Blondeau B, Garofano A, Czernichow P, Breant B . Age-dependent inability of the endocrine pancreas to adapt to pregnancy: a long-term consequence of perinatal malnutrition in the rat. Endocrinology 1999; 140: 4208–4213.
Garofano A, Czernichow P, Breant B . In utero undernutrition impairs rat beta-cell development. Diabetologia 1997; 40: 1231–1234.
Holemans K, Verhaeghe J, Dequeker J, Van Assche FA . Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J Soc Gynecol Invest 1996; 3: 71–77.
Vuguin P, Raab E, Liu B, Barzilai N, Simmons R . Hepatic insulin resistance precedes the development of diabetes in a model of intrauterine growth retardation. Diabetes 2004; 53: 2617–2622.
D'Alessio DA, Lê K-A, Tappy L . Type 2 diabetes and insulin resistance: an hepatocentric phenomenon ? Curr Opin Clin Nutr Metab Care 2005; 8: 428–430.
Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP . Early growth and coronary heart disease in later life: longitudinal study. BMJ 2001; 322: 949–953.
Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C . Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab 2000; 85: 1401–1406.
Jaquet D, Vidal H, Hankard R, Czernichow P, Levy-Marchal C . Impaired regulation of glucose transporter 4 gene expression in insulin resistance associated with in utero undernutrition. J Clin Endocrinol Metab 2001; 86: 3266–3271.
Selz R, Theintz GE, Tappy L, Schneiter P . Evaluation of hepatic and whole body glycogen metabolism in humans during repeated administrations of small loads of 13C glucose. Diabetes Metab 2003; 29: 643–649.
Selz R, Jornayvaz FR, Tappy L, Woringer V, Theintz GE . Assessment of hepatic glucose metabolism by indirect calorimetry in combination with a non-invasive technique using naturally enriched 13C glucose in healthy children and adolescents. Horm Res 2004; 62: 142–148.
Jornayvaz FR, Selz R, Tappy L, Woringer V, Theintz GE . Non-invasive assessment of whole body glycogen turnover in children and adolescents. Diabetes 2002; 51: A430.
Felber JP, Acheson KJ, Tappy L . From Obesity to Diabetes. John Wiley & Sons: Chichester, 1992.
Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD . Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 1988; 37: 1020–1024.
Crowther NJ, Trusler J, Cameron N, Toman M, Gray IP . Relation between weight gain and beta-cell secretory activity and non-esterified fatty acid production in 7-year-old African children: results from the Birth to Ten study. Diabetologia 2000; 43: 978–985.
McMillen IC, Robinson JS . Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005; 85: 571–633.
McMillen IC, Warnes KE, Adams MB, Robinson JS, Owens JA, Coulter CL . Impact of restriction of placental and fetal growth on expression of 11β-hydroxysteroid dehydrogenase type 1 and type 2 messenger ribonucleic acid in the liver, kidney, and adrenal of the sheep fetus. Endocrinology 2000; 141: 539–543.
Phillips DIW, Walker BR, Reynolds RM, Flanagan DEH, Wood PJ, Osmond C et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 2000; 35: 1301–1306.
O'Regan D, Welberg LLAM, Holmes MC, Seckl JR . Glucocorticoid programming of pituitary-adrenal function: mechanisms and physiological consequences. Semin Neonatol 2001; 6: 319–329.
Seematter G, Binnert C, Martin J-L, Tappy L . Relationship between stress, inflammation and metabolism. Curr Opin Clin Nutr Metab Care 2004; 7: 169–173.
Seematter G, Binnert C, Tappy L . Stress and metabolism. Metab Syndrome Rel Disorders 2005; 3: 8–13.
Verkauskiene R, Jaquet D, Deghmoun S, Chevenne D, Czernichow P, Lévy-Marchal C . Smallness for gestational age is associated with persistent change in insulin-like growth factor-I (IGF-I) and the ratio IGF-I/IGF binding protein-3 in adulthood. J Clin Endocrinol Metab 2005; 90: 5672–5679.
Hokken-Koelega AC, De Waal WJ, Sas TC, Van Pareren Y, Arends NJ . Small for gestational age (SGA): endocrine and metabolic consequences and effects of growth hormone treatment. J Pediatr Endocrinol 2004; 17 (Suppl 3): 463–469.
Ali O, Cohen P . Insulin-like growth factors and their binding proteins in children born small for gestational age: implication for growth hormone therapy. Horm Res 2003; 60 (Suppl 3): 115–123.
Bozzola E, Lauriola S, Messina MF, Bona G, Wasniewska M, Bozzola M et al. The risk of diabetes mellitus in children born small for gestational age and treated with recombinant growth hormone. J Pediatr Endocrinol 2005; 18: 63–67.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tappy, L. Adiposity in children born small for gestational age. Int J Obes 30 (Suppl 4), S36–S40 (2006). https://doi.org/10.1038/sj.ijo.0803517
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803517
Keywords
This article is cited by
-
Obesity in prematurely born children and adolescents: follow up in pediatric clinic
Nutrition Journal (2013)
-
The feto-placental endothelium in pregnancy pathologies
Wiener Medizinische Wochenschrift (2012)