Abstract
OBJECTIVE:
To assess the effect of weight change on the relationship between coffee and tea consumption and diabetes risk.
DESIGN:
Prospective cohort study, using data from the First National Health and Nutrition Examination Survey Epidemiologic Follow Up Study. Survival analyses were conducted using 301 selfreported cases of diabetes and eight documented diabetes deaths during an 8.4-y follow-up.
SUBJECTS:
A total of 7006 subjects aged 32–88 y with no reported history of diabetes were included in the study.
RESULTS:
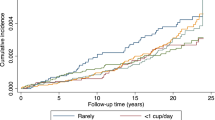
For all subjects combined, increases in consumption of ground-caffeinated coffee and caffeine at baseline were followed by decreases in diabetes risk during follow-up. There were significant statistical interactions between age and consumption of caffeine (P=0.02) and ground-caffeinated coffee (P=0.03). Age-stratified analysis showed that the decrease in diabetes risk only applied to ≤60-y-old subjects, for whom the decrease in diabetes risk also obtained for ground-decaffeinated coffee and regular tea. The multivariate hazard ratio (HR) and 95% confidence interval for a 2 cups/day increment in the intake of ground-caffeinated coffee, ground-decaffeinated coffee and regular tea was 0.86 (0.75–0.99), 0.58 (0.34–0.99) and 0.77 (0.59–1.00), respectively. The diabetes risk was negatively related to the consumption in a dose–response manner. There were strong statistical interactions between prior weight change and beverage consumption for ≤60-y-old subjects. Further analysis revealed that the decrease in diabetes risk only applied to those who had lost weight, and that there was a positive dose–response relationship between diabetes risk and weight change. For example, the multivariate HR and 95% confidence interval for >0 vs 0 cups/day of ground-decaffeinated coffee was 0.17 (0.04–0.74), 0.52 (0.19–1.42), 0.77 (0.30–1.96) and 0.91 (0.39–2.14) for subgroups with weight change of ≤0, 0–10, 10–20 and >20 lbs, respectively. There was no significant association between diabetes risk and consumption of instant-caffeinated coffee, instant-decaffeinated coffee or herbal tea. Caffeine intake appeared to explain some, but not all, of the diabetes-risk reduction and weight change.
CONCLUSION:
The negative relationship between diabetes risk and consumption of ground coffee and regular tea, observed for all NHEFS subjects, actually only applied to nonelderly adults who had previously lost weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tea Association of the USA Incorporated. Tea Fact Sheet 2004.
National Coffee Association. Coffee Drinking Trends Survey 2000.
Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD . Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21: 475–476.
Tuomilehto J, Hu G, Bidel S, Lindstrom J, Jousilahti P . Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA 2004; 291: 1213–1219.
Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB . Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 2004; 140: 1–8.
Rosengren A, Dotevall A, Wilhelmsen L, Thelle D, Johansson S . Coffee and incidence of diabetes in Swedish women: a prospective 18-year follow-up study. J Intern Med 2004; 255: 89–95.
van Dam RM, Feskens EJ . Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002; 360: 1477–1478.
Isogawa A, Noda M, Takahashi Y, Kadowaki T, Tsugane S . Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2003; 361: 703–704.
Saremi A, Tulloch-Reid M, Knowler WC . Coffee consumption and the incidence of type 2 diabetes. Diabetes Care 2003; 26: 2211–2212.
Reunanen A, Heliovaara M, Aho K . Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2003; 36: 702–703.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vendermander J . Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-hr energy expenditure and fat oxidation in humans. Am J Clin Nutr 1999; 70: 1040–1045.
Arciero PJ, Bougopoulos CL, Nindl BC, Benowitz NL . Influence of age on the thermic response to caffeine in women. Metabolism 2000; 49: 101–107.
Astrup A, Toubro S . Thermogenic, metabolic, and cardiovascular responses to ephedrine and caffeine in man. Int J Obes Relat Metab Disord 1993; 17 (Suppl 1): S41–S43.
Bracco D, Ferrarra JM, Arnaud MJ Jequier E, Schutz Y . Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol 1995; 269: E671–E678.
Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP . Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. JAMA 1995; 274: 1915–1921.
Hillar P . National Center for Health Statistics. Plan and Operation of the Health and Nutrition Examination Survey, United States – 1971–1973. National Center for Health Statistics: Hyattsville, MD; 1973. Vital and Health Statistics, Series 1, nos. 10a. & 10b. DHEW (Publication no. 73–1310).
National Center for Health Statistics. Plan and Operation of the NHANES I Epidemiologic Follow up Study 1982–1984. National Center for Health Statistics: Hyattsville, MD; 1987. Vital and Health Statistics, Series 1, No. 22. (DHHS Publication no. 87–1324).
National Center for Health Statistics. Plan and Operation of the NHANES I Epidemiologic Follow up Study 1992. National Center for Health Statistics: Hyattsville, MD, Vital and Health Statistics, Series 1, No. 35.
van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB . Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002; 136: 201–209.
US Department of Agriculture. Agriculture Handbook No. 8, Revised, Composition of Foods, Raw, Processed, Prepared. US Department of Agriculture: Washington, DC; 1967–1991.
Bunker and McWilliams'. Caffeine content of common beverages from the Caffeine content of common beverages from the Journal of the American Dietetic Association, and the list from the USDA handbook #8 1979.
Cox DR . Regression models and life tables. J Roy Stat Soc (B) 1971–1975; 34: 187–220.
SPSS. Advanced Statistics 7.5. SPSS Inc.: Chicago, IL; 1997. Pgs. 285–310.
Tabachnick BG, Fidell LS . Using Multivariate Statistics, 3rd edn, Chapter 12. Harpercollins: New York; 1996.
Johnston KL, Clifford MN, Morgan LM . Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 2003; 78: 728–733.
Greenberg JA . Biases in the mortality risk vs body mass index relationship in the NHANES-1 Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord 2001; 25: 1071–1078.
Arnlov J, Vessby B . Coffee consumption and insulin sensitivity. JAMA 2004; 291: 1199–1201.
Agardh EE, Carlsson S, Ahlbom A, Efendic S, Grill V, Hammar N, Hilding A, Ostenson CG . Coffee consumption, type 2 diabetes and impaired glucose tolerance in Swedish men and women. J Intern Med 2004; 255: 645–652.
Acknowledgements
We thank Kenneth Axen, Department of Health and Nutrition Sciences at Brooklyn College of the City University of New York, for his helpful insightful comments during the preparation of the manuscript. The original source of the NHEFS data is the National Center for Health Statistics (NCHS). The Inter-University Consortium on Political and Social Research (ICPSR) provided the data. Neither NCHSR nor ICPSR are responsible for this report, which is the work of the author, who appreciates being able to obtain and work with the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenberg, J., Axen, K., Schnoll, R. et al. Coffee, tea and diabetes: the role of weight loss and caffeine. Int J Obes 29, 1121–1129 (2005). https://doi.org/10.1038/sj.ijo.0802999
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802999
Keywords
This article is cited by
-
Dietary caffeine intake is associated with favorable metabolic profile among apparently healthy overweight and obese individuals
BMC Endocrine Disorders (2023)
-
Genetic association between coffee/caffeine consumption and the risk of obstructive sleep apnea in the European population: a two-sample Mendelian randomization study
European Journal of Nutrition (2023)
-
A systematic review to identify the effects of tea by integrating an intelligence-based hybrid text mining and topic model
Soft Computing (2021)
-
The regular consumption of coffee and development of type 2 diabetes mellitus
Journal of Public Health (2020)
-
The unmapped chemical complexity of our diet
Nature Food (2019)