Abstract
Objective: To examine long-term safety and efficacy for weight loss of an herbal Ma Huang and Kola nut supplement (90/192 mg/day ephedrine alkaloids/caffeine).
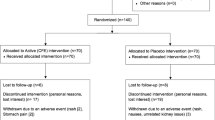
Design: Six-month randomized, double-blind placebo controlled trial.
Subjects: A total of 167 subjects (body mass index (BMI) 31.8±4.1 kg/m2) randomized to placebo (n=84) or herbal treatment (n=83) at two outpatient weight control research units.
Measurements: Primary outcome measurements were changes in blood pressure, heart function and body weight. Secondary variables included body composition and metabolic changes.
Results: By last observation carried forward analysis, herbal vs placebo treatment decreased body weight (−5.3±5.0 vs −2.6±3.2 kg, P<0.001), body fat (−4.3±3.3 vs −2.7±2.8 kg, P=0.020) and LDL-cholesterol (−8±20 vs 0±17 mg/dl, P=0.013), and increased HDL-cholesterol (+2.7±5.7 vs −0.3±6.7 mg/dl, P=0.004). Herbal treatment produced small changes in blood pressure variables (+3 to −5 mmHg, P≤0.05), and increased heart rate (4±9 vs −3±9 bpm, P<0.001), but cardiac arrhythmias were not increased (P>0.05). By self-report, dry mouth (P<0.01), heartburn (P<0.05), and insomnia (P<0.01) were increased and diarrhea decreased (P<0.05). Irritability, nausea, chest pain and palpitations did not differ, nor did numbers of subjects who withdrew.
Conclusions: In this 6-month placebo-controlled trial, herbal ephedra/caffeine (90/192 mg/day) promoted body weight and body fat reduction and improved blood lipids without significant adverse events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
NBC Nightly News In Depth Report, 10 April 2001.
Haller CA, Benowitz NL . Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids New Engl J Med 2000 343: 1833–1838.
Food and Drug Administration. Fed Reg 1997 62: 30678–30724.
Neergaard L . Since recent deaths, the FDA is studying dangers of ephedra Associated Press 17 April 2000.
Spake A . Natural hazards US News World Rep 12 February 2001 42–49.
Malchow-Moller A, Larsen S, Hey H, Stokholm KH, Juhl E, Quaade F . Ephedrine as an anorectic: the story of the ‘Elsinore pill’ Int J Obes 1981 5: 183–187.
Toubro S, Astrup AV, Breum L, Quaade F . Safety and efficacy of long-term treatment with ephedrine, caffeine and an ephedrine/caffeine mixture Int J Obes Relat Metab Disord 1993 17 (Suppl 1): S69–S72.
Astrup A, Breum L, Toubro S, Hein P, Quaade F . The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial Int J Obes Relat Metab Disord 1992 16: 269–277.
Daly PA, Krieger DR, Dulloo AG, Young JB, Landsberg L . Ephedrine, caffeine and aspirin: safety and efficacy for treatment of human obesity Int J Obes Relat Metab Disord 1993 17 (Suppl 1): S73–S78.
Breum L, Pedersen JK, Ahlstrom F, Frimodt-Moller J . Comparison of an ephedrine/caffeine combination and dexfenfluramine in the treatment of obesity. A double-blind multi-centre trial in general practice Int J Obes Relat Metab Disord 1994 18: 99–103.
Boozer C, Nasser J, Heymsfield S, Wang V, Chen G, Solomon J . An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial Int J Obes Relat Metab Disord 2001 25: 316–324.
Kaats G, Adelman J, Blum K . Effects of a multiple herbal formulation on body composition, blood chemistry, vital signs, and self-reported energy levels & appetite control Int J Obes Relat Metab Disord 1994 18: S145.
Lohman T . Advances in body composition assessment Human Kinetics: Champaigne, IL 1992.
Siri W . Body composition from fluid spaces and density: analysis of methods In Techniques for measuring body composition NAS: Washington, DC 1961 pp 223–248.
Agresti A . Categorical Data Analysis Wiley: New York 1990.
Dulloo AG, Miller DS . Reversal of obesity in the genetically obese fa/fa Zucker rat with an ephedrine/methylxanthines thermogenic mixture J Nutr 1987 117: 383–389.
Dulloo AG, Miller DS . The thermogenic properties of ephedrine/methylxanthine mixtures: animal studies Am J Clin Nutr 1986 43: 388–394.
Toubro S, Astrup A, Breum L, Quaade F . The acute and chronic effects of ephedrine/caffeine mixtures on energy expenditure and glucose metabolism in humans Int J Obes Relat Metab Disord 1993 17 (Suppl 3): S73–S77.
Dulloo AG, Miller DS . Aspirin as a promoter of ephedrine-induced thermogenesis: potential use in the treatment of obesity Am J Clin Nutr 1987 45: 564–569.
Astrup A, Toubro S, Cannon S, Hein P, Madsen J . Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study Metabolism 1991 40: 323–329.
Astrup A, Buemann B, Christensen NJ et al. The effect of ephedrine/caffeine mixture on energy expenditure and body composition in obese women Metabolism 1992 41: 686–688.
Buemann B, Marckmann P, Christensen NJ, Astrup A . The effect of ephedrine plus caffeine on plasma lipids and lipoproteins during a 4.2 MJ/day diet Int J Obes Relat Metab Disord 1994 18: 329–332.
Astrup A, Toubro S . Thermogenic, metabolic, and cardiovascular responses to ephedrine and caffeine in man Int J Obes Relat Metab Disord 1993 17 (Suppl 1): S41–S43.
Bray GA, Blackburn GL, Ferguson JM, Greenway FL, Jain AK, Mendel CM, Mendels J, Ryan DH, Schwartz SL, Scheinbauml ML, Seaton TB . Sibutramine produces dose-related weight loss Obes Res 1999 7: 189–198.
White LM, Gardner SF, Gurley BJ, Marx MA, Wang PL, Estes M . Pharmacokinetics and cardiovascular effects of ma-huang (Ephedra sinica) in normotensive adults J Clin Pharmac 1997 37: 116–122.
Ramsey JJ, Colman RJ, Swick AG, Kemnitz JW . Energy expenditure, body composition, and glucose metabolism in lean and obese rhesus monkeys treated with ephedrine and caffeine Am J Clin Nutr 1998 68: 42–51.
Karch S . Ma Huang and the ephedra alkaloids In: Cupp M (ed.) Toxicology and clinical pharmacology of herbal products Humana Press: Totowa, NJ 2000 pp 11–30.
Fleming GA . The FDA, regulation, and the risk of stroke New Engl J Med 2000 343: 1886–1887.
Hutchins GM . Dietary supplements containing ephedra alkaloids New Engl J Med 2001 344: 1095–1096.
Acknowledgements
The authors gratefully acknowledge the help of the following individuals: Wayne Snodgrass, MD, PhD, for contributing to protocol design; Stanley Heshka, PhD, for the randomization procedure; Sheila Dawling, PhD, for supervising urine toxicologic screening, Steven B Heymsfield, MD, for EKG evaluations and for supervision of physical evaluations. Research support was provided by: Science Toxicology and Technology Consulting, San Francisco, CA, USA and National Institutes of Health grant P30DK 26687.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I: medical exclusions from the study
Active heart disease, a positive history of palpitations, hypertension (office measurement ≥140 systolic BP or diastolic BP ≥90 or ABPM mean 24 h systolic BP ≥139 mmHg or diastolic BP ≥87 mmHg), epilepsy, history of mental illness, hyperthyroidism, chronic use of any drug (by self-report or by presence in urine toxicology screen) except oral contraceptives, hormone replacement therapy or synthetic thyroid hormone, active bulimia, known prostatic hypertrophy, pregnancy (reported or detected by HCG testing), glaucoma, active cancer or cancer in remission for ≤5 y, renal dysfunction, liver dysfunction (ALT, alkaline phosphatase>2×upper limit of normal), acute or chronic active hepatitis, AIDS, any acute illness within the past 4 weeks, any other chronic illness that might be adversely impacted by concurrent use of the herbal compound, concurrent participation in another research protocol involving diet or any drug use, concurrent participation in a diet program involving severe calorie restriction (800 or fewer calories per day), caffeine intake of 500 mg per day or greater, use of appetite suppressant drugs or ephedra-containing herbal supplements within the last 6 months and weight change of 5 kg or more within the past 3 months.
Appendix II: urine toxicology screen
Amphetamine metabolites, salicylates, phenothiazines, amphetamine class, barbiturates, benzodiazepines, cannabinoids, cocaine metabolites, opiates, methadone, phencyclidine, tricyclics, methanol, ethanol, acetone, iso-propanol, ethchlorvynol.
Rights and permissions
About this article
Cite this article
Boozer, C., Daly, P., Homel, P. et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes 26, 593–604 (2002). https://doi.org/10.1038/sj.ijo.0802023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802023
Keywords
This article is cited by
-
Position statement on nutrition therapy for overweight and obesity: nutrition department of the Brazilian association for the study of obesity and metabolic syndrome (ABESO—2022)
Diabetology & Metabolic Syndrome (2023)
-
Statistical analysis of two arm randomized pre-post designs with one post-treatment measurement
BMC Medical Research Methodology (2021)
-
A system for reporting and evaluating adverse drug reactions of herbal medicine in Taiwan from 1998 to 2016
Scientific Reports (2021)
-
Pharmacotherapy for childhood obesity: present and future prospects
International Journal of Obesity (2013)
-
A pilot study to evaluate the effect of Taeumjowi-tang on obesity in Korean adults: study protocol for a randomised, double-blind, placebo-controlled, multicentre trial
Trials (2012)