Abstract
BACKGROUND: We previously reported that human adenovirus Ad-36 induces adiposity and paradoxically lower levels of serum cholesterol (CHOL) and triglycerides (TG) in animals.
OBJECTIVE: To evaluate the transmissibility of Ad-36 and Ad-36 induced adiposity using a chicken model.
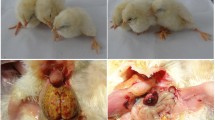
DESIGN: Experiment 1—four chickens were housed (two per cage) and one from each cage was inoculated with Ad-36. Duration of presence of Ad-36 DNA in the blood of all chickens was monitored. Experiment 2—two groups of chickens were intranasally inoculated with Ad-36 (infected donors, I-D) or media (control donors, C-D). Blood drawn 36 h later from I-D and C-D groups was inoculated into wing veins of recipient chickens (infected receivers, I-R, and control receivers, C-R, respectively). On sacrifice, 5 weeks post-inoculation, blood was drawn, body weight noted and visceral fat was separated and weighed.
RESULTS: Experiment 1—Ad-36 DNA appeared in the blood of the inoculated chickens and that of uninoculated chickens (cage mates) within 12 h of inoculation and the viral DNA persisted up to 25 days in the blood. Experiment 2—compared with C-D, visceral and total body fat were significantly greater and CHOL significantly lower for the I-D and I-R. TG were significantly lower for the I-D. Ad-36 was isolated from 12 out of 16 blood samples of the I-D that were used for inoculating I-R chickens. Ad-36 DNA was present in the blood and the adipose tissue of the I-D and I-R but not in the skeletal muscles of animals selected randomly for testing.
CONCLUSION: As seen in experiment 1, Ad-36 infection can be transmitted horizontally from an infected chicken to another chicken sharing the cage. Additionally, experiment 2 demonstrated blood-borne transmission of Ad-36-induced adiposity in chickens. Transmissibility of Ad-36-induced adiposity in chicken model raises serious concerns about such a possibility in humans that needs further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray GA . Obesity in America NIH Publication 79–359 NIH: Washington, DC 1979.
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB . A virally induced obesity syndrome in mice Science 1982 216: 82–85.
Carter JK, Garlich JD, Donaldson WT, Smith RE . Influence of diet on a retrovirus induced obesity and stunting syndrome Avian Dis 1983 27: 317–322.
Carter JK, Ow CL, Smith RE . Rous-Associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity Infect Immun 1983 39: 410–422.
Gosztonyi G, Ludwig H . Borna disease: neuropathology and pathogenesis Curr Top Microbiol Immunol 1995 190: 39–73.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA . Avian adenovirus leading to pathognomic obesity in chickens J Bombay Vet College 1990 2: 131–132.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA . Effect of adenovirus infection on adiposity in chickens Vet Microbiol 1992 31: 101–107.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL . Increased adiposity in animals due to a human virus Int J Obes Relat Metab Disord 2000 24: 989–996.
Dhurandhar NV, Augustus A, Atkinson RL . Evidence for an association of a virus with obesity in humans FASEB J 1997 3: A230.
Atkinson RL, Dhurandhar NV, Allison DB, Bowen R, Israel BA . Evidence for an association of an obesity virus with human obesity at three sites in the United States Int J Obes Relat Metab Disord 1998 22 (Suppl): S57.
Kolesar JM, Allen P, Doran CM . Direct quantification of HIV-1 RNA by capillary electrophoresis with laser-induced fluorescence J Chromatogr B 1997 697: 189–194.
Kolesar JM, Miller JA, Dhurandhar NV, Atkinson RL . Direct quantification of AD-36 adenovirus DNA by capillary electrophoresis with laser-induced fluorescence J Chromatogr B 2000 744: 1–8.
Folch J, Lees M, Sloane-Stanley YN . A simple method for the isolation and purification of total lipids from animal tissue J Biol Chem 1957 226: 497–509.
Wigand R, Gelderblom H, Wadell G . New human adenovirus (candidate adenovirus 36), a novel member of subgroup D Arch Virol 1980 64: 225–233.
Acknowledgements
We gratefully acknowledge Drs Geoffrey Letchworth and Lisa Krugner-Higby for advice on virological aspects and Sharon Gathright and Kathleen Taylor for laboratory assistance. This work was supported by funds from the Wisconsin Alumni Research Foundation's Beers-Murphy Clinical Nutrition Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhurandhar, N., Israel, B., Kolesar, J. et al. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes 25, 990–996 (2001). https://doi.org/10.1038/sj.ijo.0801668
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801668
Keywords
This article is cited by
-
Adenovirus 36 prevalence and association with human obesity: a systematic review
International Journal of Obesity (2021)
-
What we know and what we need to know about adenovirus 36-induced obesity
International Journal of Obesity (2020)
-
Ileal transcriptome analysis in obese rats induced by high-fat diets and an adenoviral infection
International Journal of Obesity (2019)
-
Viral Infections and Obesity
Current Obesity Reports (2017)
-
Longitudinal investigation of adenovirus 36 seropositivity and human obesity: the Cardiovascular Risk in Young Finns Study
International Journal of Obesity (2015)