Abstract
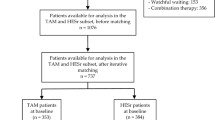
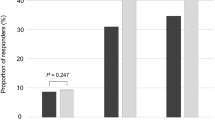
We evaluated the effects of extended-release alfuzosin HCl 10 mg once daily (q.d.) on sexual function in men with lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH). In a randomized, double-blind, placebo-controlled study of men aged ≥50 years, after a 28-day placebo run-in period, patients were randomized to receive alfuzosin 10 mg q.d. or matching placebo for 28 days. The mean change from baseline (day 1) in sexual function on day 29 was assessed using the Danish Prostate Symptom Score Sex (DAN-PSSsex) questionnaire. A total of 372 patients were randomized to receive alfuzosin (n=186) or placebo (n=186), with 355 completing the study. At baseline, 64% of the patients reported erectile dysfunction (ED) and 63% reported ejaculatory dysfunction (EjD). For the 320 patients who completed the DAN-PSSsex, alfuzosin treatment was associated with a significant improvement in the mean change from baseline in erectile function on day 29 compared with placebo (P=0.02). No significant difference was observed between the two treatment groups in the mean change from baseline in ejaculatory function on day 29. For patients with ED at baseline, a marginal improvement in erectile function was demonstrated with alfuzosin treatment (P=0.09 vs placebo). For patients with EjD at baseline, the mean change from baseline in ejaculatory function with alfuzosin was comparable to that with placebo. Dizziness was the most common adverse event with alfuzosin treatment (5 vs 0% with placebo), with other adverse events reported with comparable frequency in both treatment groups. After 1 month of treatment, alfuzosin 10 mg q.d. significantly improved erectile function in men with lower urinary tract symptoms/ benign prostatic hypertrophy and had no adverse effect on ejaculatory function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB . Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61.
Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U . Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res 2000; 12: 305–311.
Leliefeld HH, Stoevelaar HJ, McDonnell J . Sexual function before and after various treatments for symptomatic benign prostatic hyperplasia. BJU Int 2002; 89: 208–213.
Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol 2003; 44: 637–649.
Rosen RC, Giuliano F, Carson CC . Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol 2005; 47: 824–837.
van Moorselaar RJ, Hartung R, Emberton M, Harving N, Matzkin H, Elhilali M et al. Alfuzosin 10 mg once daily improves sexual function in men with lower urinary tract symptoms and concomitant sexual dysfunction. BJU Int 2005; 95: 603–608.
Mottet N, Bressolle F, Delmas V, Robert M, Costa P . Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol 2003; 44: 101–105.
van Kerrebroeck P, Jardin A, Laval KU, van Cangh P . Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37: 306–313.
Roehrborn CG . Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology 2001; 58: 953–959.
Nordling J . Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int 2005; 95: 1006–1012.
Roehrborn CG, Van Kerrebroeck P, Nordling J . Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int 2003; 92: 257–261.
Marks LS, Roehrborn CG, Gittelman M, Kim D, Forrest J, Jacobs S . First dose efficacy of alfuzosin once daily in men with symptomatic benign prostatic hyperplasia. Urology 2003; 62: 888–893.
Ahtoy P, Chretien P, Dupain T, Rauch C, Rouchouse A, Delfolie A . Alfuzosin, an alpha1-adrenoceptor antagonist for the treatment of benign prostatic hyperplasia: once daily versus 3 times daily dosing in healthy subjects. Int J Clin Pharmacol Ther 2002; 40: 289–294.
Resnick MI, Roehrborn CG . Rapid onset of action with alfuzosin 10 mg once daily in men with benign prostatic hyperplasia: a randomized placebo-controlled trial. Prostate Cancer Prostatic Dis 2007; 10: 155–159.
Schou J, Holm NR, Meyhoff HH . Sexual function in patients with symptomatic benign prostatic hyperplasia. Scand J Urol Nephrol Suppl 1996; 179: 119–122.
Hansen BJ, Flyger H, Brasso K, Schou J, Nordling J, Thorup Andersen J et al. Validation of the self-administered Danish Prostatic Symptom Score (DAN-PSS-1) system for use in benign prostatic hyperplasia. Br J Urol 1995; 76: 451–458.
FLOMAX (tamsulosin hydrochloride) [prescribing information]. Boehringer Ingelheim Pharmaceuticals Inc.: Ridgefield, CT, 2005.
Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD . Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology 2004; 64: 777–782.
Brookes ST, Donovan JL, Peters TJ, Abrams P, Neal DE . Sexual dysfunction in men after treatment for lower urinary tract symptoms: evidence from randomised controlled trial. BMJ 2002; 324: 1059–1061.
Panser LA, Rhodes T, Girman CJ, Guess HA, Chute CG, Oesterling JE et al. Sexual function of men ages 40 to 79 years: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. J Am Geriatr Soc 1995; 43: 1107–1111.
Vallancien G, Emberton M, Harving N, van Moorselaar RJ . Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol 2003; 169: 2257–2261.
Namasivayam S, Minhas S, Brooke J, Joyce AD, Prescott S, Eardley I . The evaluation of sexual function in men presenting with symptomatic benign prostatic hyperplasia. Br J Urol 1998; 82: 842–846.
Tubaro A, Polito M, Giambroni L, Famulari C, Gange E, Ostardo E . Sexual function in patients with LUTS suggestive of BPH. Eur Urol 2001; 40 (Suppl 1): 19–22.
Acknowledgements
This study was funded by sanofi-aventis. Patricia B Leinen, PhD (Tri-Med Communications, Media, PA, USA) contributed to the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosen, R., Seftel, A. & Roehrborn, C. Effects of alfuzosin 10 mg once daily on sexual function in men treated for symptomatic benign prostatic hyperplasia. Int J Impot Res 19, 480–485 (2007). https://doi.org/10.1038/sj.ijir.3901554
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901554
Keywords
This article is cited by
-
Einfluss medikamentöser BPS-Therapie auf die sexuelle Funktion
Der Urologe (2018)
-
The Effect of LUTS/BPH and Treatments on Ejaculatory Function
Current Urology Reports (2016)
-
Open label studies sufficiently demonstrate efficacy in improving sexual function
International Journal of Impotence Research (2009)
-
Sexuality and the management of BPH with alfuzosin (SAMBA) trial
International Journal of Impotence Research (2009)
-
Validated questionnaires for assessing sexual dysfunction and BPH/LUTS: solidifying the common pathophysiologic link
International Journal of Impotence Research (2008)