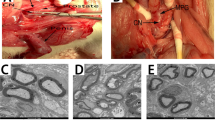
Abstract
In previous experiments, our group demonstrated morphological changes in erectile tissue from male spontaneously hypertensive rats (SHR). The present study was performed to determine whether an angiotensin II receptor blocker could protect cavernous tissue (CT) from these structural alterations in SHR. Male SHR and Wistar-Kyoto (WKY) rats were studied during 4 months. Rats were divided into three groups: SHR (n=10), SHR with candesartan cilexetil (n=10) and WKY rats (n=10). Candesartan cilexetil 7.5 mg/kg/day was administered orally throughout the study. CT was processed for pathology studies. The amount of (1) cavernous smooth muscle (CSM), (2) vascular smooth muscle (VSM), (3) collagen type III, and the rat endothelial cell antibody (RECA-1)/tunica media ratio in cavernous arteries were evaluated. SHR with candesartan cilexetil showed a lower blood pressure, a lower percentage of CSM, smaller VSM area, with a higher RECA-1/media ratio, and a lower percentage of collagen type III, when compared to untreated SHR. In addition, SHR showed a positive correlation between systolic blood pressure (SBP) and CSM amount (r=0.91; P<0.01), and SBP and the percentage of collagen type III (r=0.88; P<0.01); these correlations were not observed either in SHR treated with candesartan cilexetil or in WKY rats. We conclude that candesartan cilexetil provides a significant protective role against morphologic changes in vessels as well as in cavernous spaces of the erectile tissue, caused by high blood pressure, in SHR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feldman HA et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med 2000; 30: 328–338.
Feldman HA et al. Impotence and its medical and pshychosocial correlates: results of the male aging study. J Urol 1994; 151: 54–61.
Ledda A . Cigarette smoking, hypertension and erectile dysfunction. Curr Med Res Opin 2000; 16(Suppl 1): S13–S16.
Wei M et al. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol 1994; 140: 930–937.
Bansal S . Sexual dysfunction in hypertensive men. A critical review of the literature. Hypertension 1988; 12: 1–10.
Burchardt M et al. Erectile dysfunction is a marker for cardiovascular complications and psychological functioning in men with hypertension. Int J Impot Res 2001; 13: 276–281.
Levine LA . Diagnosis and treatment of erectile dysfunction. Am J Med 2000; 109(Suppl 9A): 3S–12S.
Burchardt M et al. Hypertension is associated with severe erectile dysfunction. J Urol 2000; 164: 1188–1191.
Johannes CB et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol 2000; 163: 460–463.
Brock GB, Lue TF . Drug induced male sexual dysfunction. Drug Safety 1993; 8: 414–426.
Toblli JE et al. Morphologic changes in cavernous tissue in spontaneously hypertensive rats. Am J Hypertens 2000; 13: 686–692.
Chitaley K, Webb RC, Dorrance AM, Mills TM . Decreased penile erection in DOCA-salt and stroke prone-spontaneously hypertensive rats. Int J Impot Res 2001; 13 (Suppl 5): S16–S20.
Gavras H . Update on the clinical pharmacology of candesartan cilexetil. Am J Hypertens 2000; 13(1 Pt 2): 25S–30S.
Virag R . Impotence: a new field in angiology. Int Angiol 1984; 3: 217–219.
Benson CB, Vickers MA . Sexual impotence caused by vascular disease: diagnosis with duplex sonography. Am J Roentgenol 1989; 153: 1149–1152.
Azadzoi KM, Goldstein I . Erectile dysfunction due to atherosclerotic vascular disease: the development of an animal model. J Urol 1992; 147: 1675–1681.
Azadzoi KM, Siroky MB, Goldstein I . Study of etiologic relationship of arterial atherosclerosis to corporal veno-occlusive dysfunction in the rabbit. J Urol 1996; 155: 1795–1800.
Azadzoi KM, Goldstein I, Siroky MB . Relationship between cavernosal ischemia and corporal veno-occlusive dysfunction in an animal model. J Urol 1997; 157: 1011–1017.
Nitahara KS, Lue TF . Microscopic anatomy of the penis, chap 4. In: Carson C, Kirby R, Goldstein I (eds). Textbook of Erectile Dysfunction. Isis Medical Media Ltd: Oxford, UK, 1999, pp 31–41.
Andersson KE, Wagner G . Physiology of penile erection. Physiol Rev 1995; 75: 191–236.
Wespes E et al. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol 1991; 146: 1015–1017.
Persson C et al. Correlation of altered penile ultrastructure with clinical arterial evaluation. J Urol 1989; 142: 1462–1468.
Jevtich MJ, Khawand NY, Vidic B . Clinical significance of ultrastructural findings in the corpora cavernosa of normal and impotent men. J Urol 1990; 143: 289–293.
Navar LG et al. Paracrine regulation of the renal microcirculation. Physiol Rev 1996; 76: 425–536.
De Nicola L, Blantz RC, Gabbai FB . Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest 1992; 89: 1248–1256.
Sigmon DH, Beierwaltes WH . Renal nitric oxide and angiotensin II interaction in renovascular hypertension. Hypertension 1993; 22: 237–242.
Kifor I et al. Tissue angiotensin II as a modulator of erectile function. I. Angiotensin peptide content, secretion and effects in the corpus cavernosum. J Urol 1997; 157: 1920–1925.
Park JK et al. Renin angiotensin system in rabbit corpus cavernosum: functional characterization of angiotensin II receptors. J Urol 1997; 158: 653–658.
Bunkenburg B, van Amelsvoort T, Rogg H, Wood JM . Receptor-mediated effects of angiotensin II on growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension 1992; 20: 746–754.
Jin XQ et al. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension 2002; 39: 1021–1027.
Ling S et al. Matrix-dependent gene expression of egr-1 and PDGF A regulate angiotensin II-induced proliferation in human vascular smooth muscle cells. Hypertension 1999; 34: 1141–1146.
Stanley AG, Patel H, Knight AL, Williams B . Mechanical strain-induced human vascular matrix synthesis: the role of angiotensin II. J Renin Angiotensin Aldosterone Syst 2000; 1: 32–35.
Laursen JB et al. Role of superoxide in angiotensin-II-induced but not catecholamine-induced hypertension. Circulation 1997; 95: 588–593.
Griendling KK et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured smooth muscle cells. Circ Res 1994; 74: 1141–1148.
Mancini JGB et al. Angiotensin converting enzyme inhibition with quinapril improves endothelial dysfunction in patients with coronary artery disease. Circulation 1996; 94: 258–265.
Prasad A et al. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation 2000; 101: 2349–2354.
Schiffrin EL et al. Correlation of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 2000; 101: 1653–1659.
Dorrance AM, Lewis RW, Mills TM . Captopril treatment reverses erectile dysfunction in stroke prone spontaneously hypertensive rats. Int J Impot Res 2002; 14: 494–497.
Hale TM, Okabe H, Heaton JPW, Adams MA . Antihypertensive drugs induce structural remodeling of the penile vasculature. J Urol 2001; 166: 739–745.
Kawamura M et al. TCV-116, a novel angiotensin II receptor antagonist, prevents intimal thickening and impairment of vascular function after carotid injury in rats. J Pharmacol Exp Ther 1993; 266: 1664–1669.
Hara K et al. Effects of TCV-116 on endothelin-1 and PDGF A-chain expression in angiotensin II-induced hypertensive rats. Hypertens Res 2001; 24: 55–64.
Kubo A et al. Inhibitory effect of an angiotensin II type 1 receptor antagonist on growth of vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol 1996; 27: 58–63.
El Mabrouk M, Touyz RM, Schiffrin EL . Differential ANG II-induced growth activation pathways in mesenteric artery smooth muscle cells from SHR. Am J Physiol Heart Circ Physiol 2001; 281: H30–H39.
Llisterri JL et al. Sexual dysfunction in hypertensive patients treated with losartan. Am J Med Sci 2001; 321: 336–341.
Acknowledgements
We thank Dr Peter Morsing, who kindly provided us with candesartan cilexetil in the present study, and the valuable contribution of Ana Uceda and Mariana Feldman, who helped with the preparation of laboratory experiments, and Jaquelina Mastantuono, who thoroughly reviewed the style of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toblli, J., Stella, I., Mazza, O. et al. Candesartan cilexetil protects cavernous tissue in spontaneously hypertensive rats. Int J Impot Res 16, 305–312 (2004). https://doi.org/10.1038/sj.ijir.3901146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901146
Keywords
This article is cited by
-
Local renin–angiotensin systems in the genitourinary tract
Naunyn-Schmiedeberg's Archives of Pharmacology (2012)
-
Valsartan treatment reverses erectile dysfunction in diabetic rats
International Journal of Impotence Research (2007)