Abstract
A recent clinical study indicated that an angiotensin II (Ang II) type 1 (AT1) receptor-neprilysin inhibitor (ARNi) designated LCZ696 (sacubitril/valsartan, as combined sodium complex) was superior to enalapril at reducing the risks of death and hospitalization due to heart failure. Therefore, we investigated the possible mechanisms of the beneficial effect of LCZ696, in which the inhibition of neprilysin enhances atrial natriuretic peptide (NP) or brain NP (ANP or BNP)-evoked signals that can block Ang II/AT1 receptor-induced aldosterone (Ald) synthesis in human adrenocortical cells. The binding affinity of valsartan+LBQ657 (active moiety of sacubitril) to the AT1 receptor was greater than that of valsartan alone in an AT1 receptor-expressing human embryonic kidney cell-based assay. There was no difference in the dissociation from the AT1 receptor between valsartan+LBQ657 and valsartan alone. In Ang II-sensitized human adrenocortical cells, ANP or BNP alone, but not LBQ657 or valsartan alone, significantly decreased Ald synthesis. The level of suppression of Ald synthesis by ANP or BNP with LBQ657 was greater than that by ANP or BNP without LBQ657. The suppression of ANP was blocked by inhibitors of regulator of G-protein signaling proteins and cyclic GMP-dependent protein kinase. The inhibition of neprilysin did not change the mRNA levels of the AT1 receptor, ANP receptor A, regulator of G-protein signaling protein, renin or 3β-hydroxysteroid dehydrogenases. In conclusion, the inhibition of neprilysin by LBQ657 enhances the NP-evoked signals that can block Ang II/AT1 receptor-induced Ald synthesis in human adrenocortical cells.
Similar content being viewed by others
Introduction
Heart failure (HF) is the end stage of all types of heart disease. The mortality due to HF can be reduced by optimal medical therapies including inhibitors of the renin–angiotensin aldosterone system and β-blockers.1, 2 The Prospective Comparison of ARNi (an angiotensin II (Ang II) type 1 (AT1) receptor-neprilysin inhibitor) with ARB on the Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) trial compared the use of ARNi (LCZ696) (sacubitril/valsartan, as combined sodium complex) in HF patients with valsartan in a parallel group study of patients with HF with a preserved ejection fraction.3 At 36 weeks, there was a reduction in left atrial volume and improvement in the New York Heart Association functional class in the LCZ696 group compared with the valsartan group. Moreover, the Prospective Comparison of ARNi with ACE Inhibitor to Determine the Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8442 patients in a double-blind manner to receive either 200 mg LCZ696 or 10 mg enalapril twice-daily in addition to the standard of care for a median follow-up of 27 months.4 In that study, LCZ696 was superior to enalapril at reducing the risks of death and hospitalization due to HF. LCZ696 was generally safe and well tolerated and showed effective blood pressure (BP) reduction in Japanese patients with hypertension and renal dysfunction without a decline in renal function.5 In addition, we recently reported that LCZ696 improved cardiac function with the reduction of fibrosis in a model of HFrEF (HF with reduced ejection fraction) in diabetic mice.6
Neprilysin is responsible for the degradation of several vasoactive peptides including atrial and brain natriuretic peptides (NPs) (ANP and BNP).7 These peptides block the negative cardiovascular effects of Ang II and aldosterone (Ald) in HF patients, including sodium retention, vasoconstriction, hypertrophy and endothelial dysfunction.8, 9, 10, 11 In addition, ANP has been shown to attenuate Ang II-induced Ald synthesis, and this ANP-mediated attenuation of Ald synthesis was blocked by inhibitors of regulator of G-protein signaling subtype 4 (RGS4) and of cyclic GMP-dependent protein kinase (PKG).12 Ald antagonism reduced both the risk of death and the risk of hospitalization among patients with systolic HF and mild symptoms.13 Thus, we hypothesized that the beneficial effects of LCZ696 observed in a clinical study may be at least partly due to the reduction of Ald synthesis through the inhibition of neprilysin independent of any blockade of the AT1 receptor.
Therefore, using human adrenocortical cells as a cell model, we investigated whether the inhibition of neprilysin enhances ANP or BNP-evoked signals that can block Ang II/AT1 receptor-induced Ald synthesis in these cells.
MATERIALS AND METHODS
Materials
The following reagents were purchased: Ang II and [Sar1, Ile8] Ang II (Peptide Institute, Osaka, Japan); ANP and BNP (Sigma-Aldrich, St Louis, MO, USA); CCG63802 (RGS4 inhibitor) (Santa Cruz Biotechnology, Dallas, TX, USA); KT5823 (PKG inhibitor) (Cayman Chemical Company, Ann Arbor, MI, USA) and 125I-[Sar1, Ile8]Ang II (PerkinElmer Japan, Tokyo, Japan). LBQ657 (an active form of sacubitril) and valsartan were kindly provided by Novartis Pharma, Basel, Switzerland.
Competition binding study
The Kd values of receptor binding were determined by 125I-[Sar1, Ile8]Ang II-binding experiments under equilibrium conditions. The AT1 receptor gene was transfected by the calcium phosphate method into HEK293 cells, and stably expressing AT1 receptor cells were selected by the addition of hygromycin B to the cells after transfection.14 Live HEK293 cells expressing the AT1 receptor were incubated with 125I-[Sar1, Ile8]Ang II and valsartan with or without LBQ657 at 37 °C in 5% CO2. Non-specific binding in the cells was determined from 125I-[Sar1, Ile8]Ang II binding in the presence of 10 μm [Sar1, Ile8]Ang II. After equilibrium was reached, the binding experiments were stopped by wash-out. The bound ligand fraction was determined from the counts per minute remaining on the cells. Equilibrium binding kinetics were determined.
Dissociation of valsartan, with or without LBQ657, and ANP from the AT1 receptor
Live HEK293 cells expressing AT1 receptors were preincubated for 90 min with each compound and further incubated with 125I-[Sar1, Ile8]Ang II in the presence of compounds or after wash-out of compounds for 30 and 90 min. % Inhibition of 125I-[Sar1, Ile8]Ang II binding to AT1 receptors was calculated for the various compounds.
Measurement of Ald synthesis
Human adrenocortical cells (NCI-H295R, American Type Culture Collection (ATCC) CRL-10296) (ATCC, Manassas, VA, USA) were grown for 24 h under serum-free conditions with or without 0.1 μm Ang II (Ang II-sensitized or -non-sensitized condition, respectively) at 37 °C in 5% CO2. After wash-out of the added Ang II, the adrenocortical cells were then incubated with or without various compounds for an additional 24 h under serum-free conditions. The levels of Ald in the cell-culture medium (Ald synthesis) after various treatments were measured in duplicate by an enzyme immunoassay (Cayman Chemical).12
Reverse transcriptase-PCR analysis
Gene expression levels were quantified by real-time PCR. In adrenocortical cells, total RNA was extracted using a RiboPure RNA Purification Kit (Life Technologies, Carlsbad, CA, USA). cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen, Germantown, MD, USA). Real-time PCR was performed with a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using a QuantiTect SYBR Green PCR Kit (Qiagen). The mRNA levels of the AT1 receptor, RGS4, ANP receptor A (NPR1), renin, two different 3β-hydroxysteroid dehydrogenases (HSDs) known as HSD3B1 and HSD3B215 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were investigated. The mRNA level of each factor (AU, arbitrary units) was determined as the ratio of each factor to GAPDH. Primer sequences are listed with the 5′ to 3′ direction written as left to right. The primers used were forward primer AGCTATGGAATACCGCTGGC and reverse primer GCGGGACTTCATTGGGTGAA for the human AT1 receptor, forward primer CAGCTGGGTTGGTATGGAGT and reverse primer GCCCTCACTCAGCCAGTAAC for human RGS4, forward primer AGTGGTGGGACTGAAGATGC and reverse primer TCGAAACCACCAAACTCCTC for human NPR1, forward primer CTCTTCGATGCTTCGGATTC and reverse primer GATGTCCTGGCTGAGAAAGC for human renin, forward primer AGAAGAGCCTCTGGAAAACACATG and reverse primer TAAGGCACAAGTGTACAGGGTGC for human HSD3B1, forward primer AGAAGAGCCTCTGGAAAACACATG and reverse primer CGCACAAGTGTACAAGGTATCACCA for human HSD3B2, forward primer CCCATGTTCGTCATGGGTGT and reverse primer TGGTCATGAGTCCTTCCACGATA for human GAPDH.
Statistical analysis
The results are expressed as the mean±s.d. of three or more independent determinations. The significance of differences among measured values was evaluated with an analysis of variance using Fisher’s t-test and an unpaired or paired Student’s t-test, as appropriate. Statistical significance was set at <0.05.
Results
Binding affinities of valsartan and valsartan+LBQ657 in an AT1 receptor-expressing HEK cell-based living assay
The binding affinity of valsartan+LBQ657 was greater than that of valsartan alone in an AT1 receptor-expressing HEK cell-based assay using live cells (Figure 1a). The difference in Kd values between valsartan+LBQ657 (4.8±0.7 nm) and valsartan alone (6.9±0.7 nm) was small.
Dissociation of valsartan, with or without LBQ657, and ANP from the AT1 receptor
Figure 1b shows the dissociation of valsartan, with or without LBQ657, and ANP from the AT1 receptor. There were no differences in the dissociation from the AT1 receptor among valsartan alone, valsartan+LBQ657, valsartan+ANP and valsartan+LBQ657+ANP.
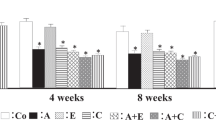
Percentages (%) of Ald synthesis in Ang II-sensitized and -non-sensitized adrenocortical cells with or without treatment with LBQ657, ANP and valsartan
The percent Ald synthesis in Ang II-sensitized and -non-sensitized adrenocortical cells is shown in Figure 2. In this figure, 100% Ald synthesis indicates the basal level of Ang II-sensitized cells without any treatment. The basal level of Ald synthesis in Ang II-non-sensitized cells was approximately 70%. ANP+valsartan both with and without LBQ657 significantly suppressed the basal level of Ald synthesis in Ang II-non-sensitized cells. In Ang II-sensitized cells, ANP both with and without valsartan significantly suppressed Ald synthesis. In addition, comparing results in Ang II-sensitized cells, ANP+valsartan with LBQ657 significantly suppressed Ald synthesis compared with ANP+valsartan without LBQ657.
Percentages (%) of aldosterone (Ald) synthesis from adrenocortical cells with or without LBQ657, ANP and valsartan. In this figure, 100% Ald synthesis indicates the basal level of Ang II-sensitized cells without treatment. Ang II-sensitized or -non-sensitized cells were grown for 24 h under serum-free conditions with or without 0.1 μm Ang II before various treatments, respectively. *P<0.05 vs. Ang II-non-sensitized cells with no treatment; tp<0.05 vs. Ang II-sensitized cells with no treatment; #P<0.05 vs. Ang II-sensitized cells with ANP+valsartan.
Percentages (%) of Ald synthesis in Ang II-sensitized adrenocortical cells with or without various concentrations of LBQ657 and valsartan
There were no differences in the % Ald synthesis under various concentrations of LBQ657 and valsartan in Ang II-sensitized cells (Figure 3a).
(a) Percentages (%) of aldosterone (Ald) synthesis in Ang II-sensitized adrenocortical cells with or without various concentrations of LBQ657 and valsartan. In this figure, 100% Ald synthesis indicates the basal level of Ang II-sensitized cells without treatment. (b) Percentages (%) of aldosterone (Ald) synthesis in Ang II-sensitized adrenocortical cells with or without LBQ657 or ANP and BNP. In this figure, 100% Ald synthesis indicates the basal level of Ang II-non-sensitized cells without treatment. *P<0.05 vs. Ang II-sensitized cells with no treatment. #P<0.05. Ang II-sensitized or -non-sensitized cells were grown for 24 h under serum-free conditions with or without 0.1 μm Ang II before various treatments, respectively.
Percentages (%) of Ald synthesis in Ang II-sensitized adrenocortical cells with or without LBQ657 or ANP and BNP
In Ang II-sensitized cells, both ANP and BNP alone, but not LBQ657 or valsartan alone, significantly decreased Ald synthesis (Figure 3b). The suppression of Ald synthesis by ANP or BNP combined with LBQ657 was greater than the suppression observed for ANP or BNP without LBQ657.
Percentages (%) of Ald synthesis in Ang II-sensitized adrenocortical cells with or without KT5823 and CCG63802
In Ang II-sensitized cells, ANP alone significantly suppressed Ald synthesis. The suppression of ANP was blocked by inhibitors of RGS proteins (CCG63802) and of PKG (KT5823) (Figure 4).
Percentages (%) of aldosterone (Ald) synthesis from Ang II-sensitized adrenocortical cells with or without KT5823 and CCG63802. In this figure, 100% Ald synthesis indicates the basal level of Ang II-non-sensitized cells without treatment. Ang II-sensitized or -non-sensitized cells were grown for 24 h under serum-free conditions with or without 0.1 μm Ang II before various treatments, respectively. *P<0.05 vs. Ang II-sensitized cells with no treatment; #P<0.05.
mRNA levels in Ang II-sensitized adrenocortical cells with or without various treatments
The inhibition of neprilysin by LBQ657, ANP and BNP did not change the mRNA levels of the AT1 receptor, RGS4, NPR1, renin, HSD3B1 and HSD3B2 (Figure 5).
mRNA ratios of the AT1 receptor/GAPDH, RGS4/GAPDH, NPR1/GAPDH, renin/GAPDH, HSD3B1/GAPDH and HSD3B2/GAPDH in Ang II-sensitized adrenocortical cells with or without various treatments. Ang II-sensitized cells were grown for 24 h under serum-free conditions with 0.1 μm Ang II before various treatments. AU, arbitrary units; NS, not significant.
Discussion
In the present study, we confirmed that the inhibition of neprilysin by LBQ657 enhanced the NP-evoked signals that can block Ang II/AT1 receptor-induced Ald synthesis in human adrenocortical cells.
We hypothesized that the beneficial effects of LCZ696 in a clinical study could be at least partly due to the reduction of Ald synthesis through the inhibition of neprilysin independent of any blockade of the AT1 receptor. In addition, we previously indicated that LCZ696-induced improvement of cardiac function in the HFrEF model in diabetic mice may be due to the specific inhibition of neprilysin, beyond the ARB effect of LCZ696.6 In this study, we found that the suppression of Ald synthesis by ANP or BNP with LBQ657 was greater than that by ANP or BNP without LBQ657. The inhibition of the degradation of NPs has been evaluated for potential therapeutic benefits. Neprilysin is the major enzyme that contributes to extracellular degradation.16 Because LBQ657 is a specific inhibitor of neprilysin, it has beneficial effects. In humans, omapatrilat inhibits both neprilysin and angiotensin-converting enzyme and increases urinary levels of ANP.17 In a preclinical study, AHU377, which is a prodrug for active LBQ657, increased cyclic GMP levels, natriuresis and diuresis in response to exogenous administration of ANP.18 Oral administration of LCZ696 increased ANP levels in a dose-dependent manner.18 Thus, the elevation or maintenance of ANP levels through the inhibition of neprilysin could also decrease Ald synthesis in vivo because the present in vitro study found that LBQ657 with either ANP or BNP suppressed Ald synthesis as a beneficial effect. On the other hand, other vasoactive peptides in addition to NPs have been identified as neprilysin substrates, including Ang I and II.19 The inhibition of neprilysin increases the levels of Ang I and II and this increase has undesirable effects, such as vasoconstriction.20 In our experiment, we obtained Ang II-sensitized adrenocortical cells by pre-incubating the cells with Ang II for 24 h. The level of Ald in cell-culture medium (Ald synthesis) after various treatments for 24 h was measured after wash-out of the cells. As a result of the wash-out, the undesirable effects alluded to earlier may not occur. Even if the inhibition of neprilysin increases the level of Ang II, with the use of LCZ696, the undesirable effects of Ang II may be simultaneously prevented by valsartan.
There are several possible mechanisms by which NPs can block Ald synthesis. We found that the ANP-mediated attenuation of Ald synthesis was blocked by CCG63802 (RGS4 inhibitor) and KT5823 (PKG inhibitor). Guanylyl cyclase-A, an NP receptor, activates a PKG-RGS4 pathway, which attenuates a Galpha(q)-dependent pathway.21 In this study, we found that Ang II-sensitized Gq-dependent Ald synthesis was blocked by ANP-induced activation of a PKG-RGS4 pathway. To confirm the mechanism, we need to measure Ald synthesis using RGS4-transfected or knockdown cells and RGS4-transgenic or knockout mice. Another possible mechanism for the attenuation of Ald synthesis by ANP is that ANP may block Ald synthesis in the biosynthetic pathway from cholesterol to pregnenolone and from corticosterone to Ald.22, 23 Although there were no significant changes in mRNA levels of HSD3B1 or HSD3B2 in this study, we did not analyze all of the enzymes in the relevant steroid hormone pathway. Further studies will be needed to resolve these issues.
The binding affinity of valsartan+LBQ657 was greater than that of valsartan alone. Although we do not know the mechanisms at this time, we used an AT1 receptor-expressing HEK cell-based live assay. LBQ657, a neprilysin inhibitor, sustains biologically-active NPs in addition to endothelin and calcitonin gene-related peptides.24 These factors might affect the changes in the structure and function of the AT1 receptor.
In conclusion, this study demonstrated that the inhibition of neprilysin by LBQ657 enhances NP-evoked signals that can block Ang II/AT1 receptor-induced Ald synthesis in human adrenocortical cells. Although our findings are based on basic research, they may lead to exciting insights into the beneficial effects of LCZ696 in the reduction of Ald synthesis through the inhibition of neprilysin in a clinical setting.
References
Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARMAlternative trial. Lancet 2003; 362: 772e6.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH . The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334: 1349–1355.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ . Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380: 1387–1395.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition vs. enalapril in heart failure. N Engl J Med 2014; 371: 993–1004.
Ito S, Satoh M, Tamaki Y, Gotou H, Charney A, Okino N, Akahori M, Zhang J . Safety and efficacy of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction. Hypertens Res 2015; 38: 269–275.
Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K . LCZ696, an angiotensin-receptor neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail 2016; 18: 386–393.
Potter LR . Natriuretic peptide metabolism, clearance and degradation. FEBS J 2011; 278: 1808e17.
Dell’Italia LJ . Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circ Res 2011; 109: 437e52.
He BJ, Anderson ME . Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab 2013; 24: 21e30.
Mulè G, Nardi E, Guarino L, Cacciatore V, Geraci G, Calcaterra I, Oddo B, Vaccaro F, Cottone S . Plasma aldosterone and its relationship with left ventricular mass in hypertensive patients with early-stage chronic kidney disease. Hypertens Res 2015; 38: 276–283.
Cuspidi C, Tadic M, Sala C . Aldosterone and abnormal left ventricular geometry in chronic kidney disease. Hypertens Res 2015; 38: 314–316.
Miura S, Nakayama A, Tomita S, Matsuo Y, Suematsu Y, Saku K . Comparison of aldosterone synthesis in adrenal cells, effect of various AT1 receptor blockers with or without atrial natriuretic peptide. Clin Exp Hypertens 2015; 37: 353–357.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.
Ishida J, Asada S, Daitoku H, Fujiwara K, Kon Y, Sugaya T, Murakami K, Nakajima T, Kasuya Y, Fukamizu A . Expression and characterization of mouse angiotensin II type 1a receptor tagging hemagglutinin epitope in cultured cell. Int J Mol Med 1999; 3: 263–270.
Ota T, Doi M, Yamazaki F, Yarimizu D, Okada K, Murai I, Hayashi H, Kunisue S, Nakagawa Y, Okamura H . Angiotensin II triggers expression of the adrenal gland zona glomerulosa-specific 3β-hydroxysteroid dehydrogenase isoenzyme through de novo protein synthesis of the orphan nuclear receptors NGFIB and NURR1. Mol Cell Biol 2014; 34: 3880–3894.
Sonnenberg JL, Sakane Y, Jeng AY, Koehn JA, Ansell JA, Wennogle LP, Ghai RD . Identification of protease 3.4.24.11 as the major atrial natriuretic factor degrading enzyme in the rat kidney. Peptides 1988; 9: 173–180.
Massien C, Azizi M, Guyene TT, Vesterqvist O, Mangold B, Menard J . Pharmacodynamic effects of dual neutral endopeptidase angiotensin-converting enzyme inhibition vs. angiotensin converting enzyme inhibition in humans. Clin Pharmacol Ther 1999; 65: 448–459.
Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP . Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol 2010; 50: 401–414.
Stephenson SL, Kenny AJ . Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J 1987; 241: 237–247.
Ferro CJ, Spratt JC, Haynes WG, Webb DJ . Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 1998; 97: 2323–2330.
Tokudome T, Kishimoto I, Horio T, Arai Y, Schwenke DO, Hino J, Okano I, Kawano Y, Kohno M, Miyazato M, Nakao K, Kangawa K . Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation 2008; 117: 2329–2339.
Campbell WB, Currie MG, Needleman P . Inhibition of aldosterone biosynthesis by atriopeptins in rat adrenal cells. Circ Res 1985; 57: 113–118.
Olson LJ, Ho BY, Cashdollar LW, Drewett JG . Functionally active catalytic domain is essential for guanylyl cyclase-linked receptor mediated inhibition of human aldosterone synthesis. Mol Pharmacol 1998; 54: 761–769.
McDowell G, Coutie W, Shaw C, Buchanan KD, Struthers AD, Nicholls DP . The effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptides. Br J Clin Pharmacol 1997; 43: 329–332.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
KS is a Chief Director and SM is a Director of NPO Clinical and Applied Science, Fukuoka, Japan. KS has an Endowed Department, the ‘Department of Molecular Cardiovascular Therapeutics,’ that is supported by MSD, Co. Ltd. SM belongs to the Department of Molecular Cardiovascular Therapeutics supported by MSD, Co. Ltd.
Rights and permissions
About this article
Cite this article
Miura, SI., Suematsu, Y., Matsuo, Y. et al. The angiotensin II type 1 receptor-neprilysin inhibitor LCZ696 blocked aldosterone synthesis in a human adrenocortical cell line. Hypertens Res 39, 758–763 (2016). https://doi.org/10.1038/hr.2016.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.72
Keywords
This article is cited by
-
The renin-angiotensin-aldosterone system: a new look at an old system
Hypertension Research (2023)
-
Aldosterone breakthrough from a pharmacological perspective
Hypertension Research (2022)
-
Impact of mineralocorticoid receptor blockade with direct renin inhibition in angiotensin II-dependent hypertensive mice
Hypertension Research (2020)
-
Greater reductions in plasma aldosterone with aliskiren in hypertensive patients with higher soluble (Pro)renin receptor level
Hypertension Research (2018)
-
Dual inhibitory action on aldosterone by combined angiotensin receptor antagonism and neprilysin inhibition
Hypertension Research (2016)