Abstract
Little information is available concerning the association between sleep quality and blood pressure (BP) in Chinese individuals. This study evaluated the association between sleep quality, as determined by the Pittsburgh sleep quality index (PSQI), and hypertension in a rural Chinese population. Using a multistage cluster and random sampling method, a representative sample of 9404 adults aged 20–93 years in northeastern China was selected from 2012 to 2013. Sleep quality was assessed by PSQI, and trained observers measured BP. A sleep disorder (SD) was diagnosed for any participant with a score of 6 or greater. Overall, 1218 male participants (25.53%) and 1261 female participants (27.22%) were defined as having SDs. Compared with the normal subjects, participants with hypertension had higher global PSQI scores and subscores in all elements. The odds ratios (ORs) of hypertension, systolic hypertension and diastolic hypertension among participants with SDs were 2.38 (95% confidence interval (CI): 2.13–2.65), 2.52 (95% CI: 2.26–2.80) and 1.93 (95% CI: 1.74–2.14) in contrast to the reference group, respectively. The risk for hypertension in poor sleepers with subscores over 0 in all of the elements was significantly increased, with ORs ranging from 1.16 (95% CI: 1.04–2.30) to 3.88 (95% CI: 1.24–12.16). The global PSQI score and its components were associated with hypertension and high BP.
Similar content being viewed by others
Introduction
Hypertension affects ~26% of adults across the world. It contributes to 13.5% of deaths worldwide and is the leading risk factor for mortality.1 The prevalence of hypertension is projected to increase and to affect over 1.5 billion people by 2025. High blood pressure (BP) leads to half of all strokes and ischemic heart disease events.2 The major risk factors for hypertension have been established and include family history, sedentary lifestyle, poor diet, cigarette smoking, sex, race and age. An additional risk factor that is unconventional and often overlooked is sleep.3 A study conducted by the Centers for Disease Control and Prevention reported that 11% of adults in the United States received insufficient rest or sleep.4 Sleep serves to suppress BP by decreasing the secretion of catecholamine.5 However, the association of sleep duration with hypertension is inconsistent.6, 7, 8, 9, 10, 11 Some studies have reported a positive relationship between poor sleep quality (apnea and insomnia) and hypertension.6, 7, 8, 9, 10 However, other studies have not shown a link between sleep duration and hypertension.11
Urban populations have been most the common sources for studies on the association between sleep quality and hypertension to date. However, people living in rural areas make up a large portion of the total population, especially in developing countries such as China. Approximately 57% of all Chinese people lived in rural agricultural regions in 2005.12 However, limited data exist on sleep quality and hypertension. Wang et al. have reported that High BP is associated with short sleep duration in women but is associated with long sleep duration in men among Chinese adults living in rural areas.13
To date, findings of previous studies on the association between sleep and hypertension have been limited by methodological variations in study populations and study designs, small sample sizes and a focus primarily on one sleep abnormality at a time and its relationship with hypertension. Moreover, it is challenging to compare findings among different studies because of the varying instruments used to measure sleep or sleep quality. Findings from previous studies have also been limited by residual confounding without consideration of factors such as gender and lifestyle choices, including alcohol consumption and physical activity.
To address these gaps in the literature, our objective in this study was to investigate the association between sleep quality and hypertension while adjusting for relevant confounders in a large rural Chinese sample. We hypothesized that low sleep quality is associated with an increased likelihood of hypertension. Our study was based on self-reported, validated measurements of sleep quality with the Pittsburgh Sleep Quality Index (PSQI).
Materials and Methods
Study population
We conducted population-based cross-sectional surveys using a multistage, stratified clustering sampling scheme in the rural areas of Jinzhou, Liaoning province, in northeastern China from 2012 to 2013. The total population in Jinzhou is ~3 102 000. In our sampling strategy, the country was stratified into five geographic zones: North, South, East, West and Central. Two townships were randomly selected from each of the South, East and West zones and one township was selected from each of the North and Central zones. Three or five villages were subsequently randomly selected from each township, which yielded 35 villages in total. During the last stage of the survey sampling, participants were stratified into several sex and age groups in accordance with census year 2010 in China. One participant aged ⩾20 years with 5 years or more of residency at the current address was selected from each household without replacement.
A total of 10 759 individuals were randomly selected from the selected villages, and 9404 persons (87% response rate; 4770 males and 4634 females) completed the survey and examination. The ages of the participants ranged from 20 to 93 years, with an average age of 52.11±14.10 years.
We performed the study procedures in accordance with the ethical standards of the responsible committee on human experimentation of Sun Yat-sen University, and the ethics committees and other relevant regulatory bodies in Liaoning province approved the study. We obtained written informed consent from each participant before data collection.
Measures
The primary outcome of interest in the present study was hypertension. For the ascertainment of hypertension, all investigators and staff of the current study were required to successfully complete a training program based on the procedures created by the American Heart Association14 to execute the administration of the study survey and the standardized protocol to measure BP. After the completion of the training program, each successful trainee was required to pass a qualifying examination; those who were successful were given a BP observer certificate. Study participants were told not to smoke, drink alcohol, drink coffee, drink tea and/or exercise for at least 30 min before having their BP measured. Four different sizes of BP cuffs (pediatric cuff, regular adult cuff, thigh cuff and large adult cuff) were placed on the individual to determine the appropriate cuff size on the basis of the individual’s arm circumference. A trained and certified observer measured BP with the participant in a seated position every 2 min for 6 min, using a standardized mercuric-column sphygmomanometer. The three BP measurements were averaged to determine the subject's BP. All of the measurements were taken during one visit. We defined hypertension as an average systolic BP of ⩾140 mm Hg, an average diastolic BP of ⩾90 mm Hg or self-reported current treatment for hypertension with antihypertensive medication according to the Seventh Report of the Joint National Committee.15 Medication use was ascertained by means of a standardized interview. We defined systolic hypertension as systolic BP ⩾140 mm Hg, and diastolic hypertension was defined as diastolic BP ⩾90 mm Hg.16
Sleep quality
We assessed sleep quality by the PSQI scale,17 which is a well validated and widely used measure of sleep quality.18, 19, 20, 21 The questionnaire had previously been translated into Chinese and had been used in previous studies in other Chinese areas.18, 19, 20, 21 PSQI is a questionnaire that consists of 19 items that are coded on a four-point scale (0–3) to obtain seven elements: subjective sleep quality (a subjective feeling of satisfaction in daily sleep), sleep disturbance (interruption of sleep), daytime dysfunction (trouble staying awake while engaging in social activity), sleep onset latency (length of time for transition from full wakefulness to sleep), habitual sleep efficiency (proportion of hours slept to total hours in bed), sleep duration and use of sleep medication.22 The sum of all subscale scores represents the total sleep quality score ranging between 0 and 21, with higher scores representing poorer sleep quality. According to the definition from other studies, any participant with a score of 6 or greater was considered to have poor sleep quality.18, 22, 23 We assessed both the global PSQI score and the seven components listed above by the four-grade system (that is, 0, 1, 2 and 3).17 Each component was assessed on a point scale (with descriptions in parentheses) as follows: subject sleep quality, 0–3 points (0, very good sleep quality; 1, fairly good sleep quality; 2, fairly bad sleep quality; and 3, very bad sleep quality); sleep latency 0–3 points (minutes required to transition from full wakefulness to sleep each night and the frequency of falling asleep within 30 min); sleep duration 0–3 points (0, sleep duration >7 h; 1, 6 to ⩽7 h; 2, 5 to <6 h and 3, <5 h); habitual sleep efficiency 0–3 points (proportion of hours slept to hours spent in bed: 0, ⩾85%; 1, 75–84%; 2, 65–74% and 3, <65%); sleep disturbance 0–3 points (sleep disruption and frequency of disruption); use of sleep medication 0–3 points (0, not used during the past month; 1, less than once a week; 2, once or twice a week and 3, three or more times a week); and daytime dysfunction 0–3 points (frequency of having trouble staying awake while driving, eating meals, or engaging in social activity and frequency of having trouble keeping up enough enthusiasm to get things done).17
Covariates
Information regarding demographic characteristics, such as age, gender, education, ethnicity, occupation, household income, and behavioral and lifestyle risk factors, including smoking, alcohol consumption, tea consumption and exercise, were collected via a self-reported questionnaire. We collected a history of hypertension and use of antihypertensive medication by using a standard questionnaire. Trained study staff recorded the weight and height of the participants and calculated the body mass index (BMI; weight in kg per height in m2). We defined alcohol consumption as the weekly consumption of beer, wine and hard liquor. Current drinking was defined as alcohol consumption ⩾8 ml per week according to the definition from the National Institute on Alcohol Abuse and Alcoholism.24 We defined smokers as people who smoked at least one cigarette per day and continued for at least 1 year. We asked whether they currently smoked (Do you smoke currently?). Tea consumption and exercise were defined as people who drank tea or exercised almost daily throughout the past year.
Statistical analysis
Baseline characteristics were compared between participants with and without poor sleep quality using Pearson χ2 tests for nominal variables (that is, gender, drinking, smoking, tea consumption, exercise or hypertension) and one-way analysis of variance for continuous variables (that is, age, BMI, systolic BP, diastolic BP and/or global PSQI score). To assess the association of self-reported sleep quality with hypertension, we performed analysis of covariance and logistic regression. We conducted data analysis of covariance for continuous variables to determine any significant differences among groups with or without hypertension adjusted for age, gender, drinking, smoking, tea consumption habit and exercise. Logistic regression was conducted to estimate the odds ratios and the corresponding 95% confidence intervals (95% CIs) for the associations between hypertension and poor sleep quality using the global PSQI score and seven other PSQI component subscales after adjusting for the aforementioned variables.22 Further logistic regression analyses were performed for participants with systolic and diastolic hypertension to estimate the odds ratio and 95% CI of sleep quality as a function of the increased risk for systolic hypertension or diastolic hypertension after adjusting for the aforementioned confounders.
All analyses were conducted using SPSS 18.0 J (SPSS Inc., Chicago, IL, USA) for Windows, and P-values<0.05 were considered to be statistically significant.
Results
The characteristics of the study sample are presented in Table 1 and are stratified by sleep quality defined by a global PSQI score ⩽5 (good sleep quality, non-SD) and a global PSQI score ⩾6 (poor sleep quality). Among the 9404 study participants (49.28% female; mean age: 52.11 years; SD: 14.10 years; range: 20–93 years), we identified 2479 individuals (26.36%) as having poor sleep quality and 4800 individuals with hypertension.
There were significant differences in sleep quality in different groups on the basis of age, BP and smoking habits. The mean age for subjects with poor sleep quality was 56.38 years, which was significantly higher than the mean age in subjects with good sleep quality (50.58 years, P<0.001). The prevalence of hypertension in subjects with poor sleep quality was high (69.74%) and substantially greater than compared with subjects with good sleep quality (44.35%, P<0.001). The proportion of participants with smoking habits was greater in subjects with poor sleep quality compared with the proportion of subjects with good sleep quality (34.25 and 28.94%, respectively; P<0.001). Hypertensive subjects had a higher prevalence of poor sleep quality than study subjects without hypertension (36.02% vs 16.29%; P<0.001; Figure 1).
Table 2 shows the significant differences among the groups of individuals with and without hypertension. The mean score for the global PSQI score and PSQI sub-component scores of all of the elements (sleep duration, subjective sleep quality, habitual sleep efficiency, sleep latency, sleep disturbance, use of sleep medication and daytime dysfunction) were significantly higher in participants with hypertension compared with subjects without hypertension (P<0.001).
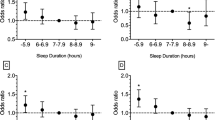
The results from the multivariable logistic regression analysis on the associations between the global PSQI and its components and hypertension after adjusting for age, gender and lifestyle factors are shown in Table 3. The odds of hypertension among subjects with a global PSQI score of 6 or more was 2.38 (95% CI, 2.13–2.65) compared to subjects whose PSQI global scores were 5 or less. Overall, study participants who had elevated scores in each of the sub-components of the PSQI had greater odds of hypertension than those who did not. For instance, the odds of hypertension among the subjects with subjective sleep quality scores of 1, 2 and 3 (indicating poorer sleep quality) were 1.82, 2.46 and 1.91 (95% CI: 1.65–2.0, 2.00–3.03 and 1.22–2.99), respectively, in contrast with the reference group.
To understand how sleep quality affects hypertension, logistic regression analysis was conducted to determine the associations between poor sleep quality (based on global PSQI), systolic hypertension and diastolic hypertension after adjusting for age, gender and lifestyle factors. The results are shown in Table 4. The odds of systolic hypertension among participants with a PSQI score of 6 or more total global points was 2.52 (95% CI: 2.26–2.80), in contrast to subjects with 5 or fewer points, and that of diastolic hypertension was 1.93 (95% CI: 1.74–2.14). Thus, poor sleep quality was associated with increased odds of both systolic and diastolic hypertension.
Discussion
Sleep duration has been a focus in previous studies on sleep health. Sleep duration measurements alone are insufficient to elucidate how sleep may affect health. Sleep quality and other sleep parameters should also be considered. However, there has been a lack of attention given to global sleep status, including sleep latency, habitual sleep efficiency, sleep disturbance and daytime dysfunction. Unlike the majority of previous investigations, we used the PSQI scale to evaluate global sleep status. We examined the associations between hypertension and the global PSQI scale and its components. We observed significant associations between hypertension and global PSQI scores, poor sleep quality, prolonged sleep latency, short sleep duration, sleep disturbance and daytime dysfunction. To date, few studies have reported an association between hypertension and PSQI components in rural populations. The current study indicates that PSQI components including poor sleep quality, prolonged sleep latency, short sleep duration and sleep disturbance are associated with hypertension, thus indicating the importance of sleep quality and other components for the comprehensive understanding of sleep. Moreover, few studies on the relationship between sleep and hypertension have focused on rural populations. Because hypertension is a prevalent problem in rural regions in China, the present study included a large sample of residents from the rural areas of north China. The present study is one of the first to obtain self-reported sleep quality assessments from a large sample of rural adults anywhere and to show associations between poor sleep quality and the risk of hypertension in the general population.
Both Huanga et al. and Erden et al. have reported that poor sleep quality is an independent predictor of nondipping hypertension (defined as the absence of a nocturnal reduction in systolic BP of ⩾10%).25, 26, 27 Fiorentini et al. also have reported that poor sleep quality evaluated by the PSQI is associated with hypertension.10 However, some studies have reported that there is no association between the global PSQI score and hypertension.8, 28 One possible explanation for this discrepancy is that these studies included both healthy individuals and patients with a limited age range. In addition, some potential confounders were not previously taken into account, including age, gender, BMI, alcohol consumption, smoking, tea consumption and exercise habits.10, 29 We controlled for these potential confounders in this study. The participants of this study were all rural Chinese adults, and the mean of their global PSQI score for individuals with and without hypertension were both less than 6 (that is, they had no SDs).30 However, the global PSQI scores for the subjects with hypertension were higher than those without hypertension. Consequently, participants with high global PSQI scores would be at higher risk for hypertension, even though they did not have SDs.22
Currently, the mechanisms underlying the relationship between sleep and BP regulation are not fully understood. Sympathetic nerve activity to the vasculature has been reported to play an important role in the relationship between sleep and hypertension.31 People exhibit non-significant changes in cardiac output during the night. A decrease in total peripheral vascular resistance leads to the normal nocturnal drop in arterial pressure (‘dipping’). Sleep sympathetic activity significantly increases and is highly variable during rapid eye movement (REM) compared with the waking hours. The BP approaches wakefulness levels during the phasic component of REM, and baroreflex sensitivity increases during sleep. However, it is more effective to buffer increases in BP during REM episodes occurring at the end of the sleep period than earlier in the night. Thus, the regulation of nocturnal BP could be linked to sleep characteristics. Abnormal sleeping is involved in the pathogenesis of nondipping prehypertension and subsequently in hypertension disturbances in sleep quality leads to hypertension.31 Habitual sleep problems also lead to the development and maintenance of hypertension.28 This phenomenon may be due to waking physical and psychosocial stressors, elevated sympathetic nervous system activity, changes in exposure to raised 24-h BP and heart rate, and increased salt retention. Dramatic changes in these factors in modern industrialized society are thought to cause the environment that is recognized by the brain to become metabolically flattened and arrhythmic, thereby disturbing the circadian rhythmicity of BP in high-risk individuals.28 Huang et al. also reported that poor sleep quality is an important risk factor for hypertension possibly because of higher activation of the sympathetic nervous system.29 In the present study, it was confirmed that not only poor sleep quality but also other sleep problems including disturbance in sleep quantity or quality are associated with the prevalence of hypertension. Furthermore, the present study also found that adults with poor sleep quality are more likely to have systolic hypertension, which is related to sympathetic nerve activity.
Consistently with prior research, the present study observed that short sleep duration is associated with hypertension. Some previous studies also reported this association between sleep duration and hypertension.23, 31, 32, 33, 34 Our study has a number of strengths. We were able to control for a number of confounding variables in the association between sleep and hypertension. In addition, we evaluated both the sleep duration and a comprehensive number of sleep components, including sleep quality, habitual sleep efficiency, sleep latency and sleep disturbance. Our results show that sleep duration was correlated with other PSQI components. Interestingly, our data indicate that the effect of poor sleep quality on hypertension is larger than that of sleep duration.
However, our study has some limitations. First, we could not demonstrate any causal relationship between hypertension and sleep status, because of the nature of the cross-sectional study. The participants might have changed their diets and the conditions related to elevated hypertension and sleep habits. However, the lifestyles, sleep habits and food habits of this population were relatively stable and similar. Second, we used self-reported brief questionnaires to evaluate sleep-related factors. Sleep evaluation based on a combination of polysomnography and self-reported questionnaires might have provided a different result.22 Third, we did not exclude obstructive sleep apnea, which would trigger impaired sleep quality and sleep fragmentation and enhance stress hormone secretion. Fourth, serum creatinine was not measured in this study, and therefore an estimated glomerular filtration rate could not be calculated. Given the strong association between reduced estimated glomerular filtration rate and hypertension, this is a major limitation. Finally, we did not adjust for other potential confounding factors, such as socio-economic status and a family history of SDs.
Conclusion
The findings suggest that a high global PSQI score is associated with increased odds of hypertension in a rural Chinese population. Increased hypertension is also associated with PSQI components including short sleep duration, poor sleep quality, prolonged sleep latency and sleep disturbance. A prospective population-based cohort study is needed in the future to validate this finding, and more potential confounding factors should be considered.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Lawes CM, Vander Hoorn S, Rodgers A . International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371: 1513–1518.
WRITING GROUP MEMBERS, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation 2010; 121: e46–e215.
Centers for Disease Control Prevention. Perceived insufficient rest or sleep among adults—United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58: 1175–1179.
Portaluppi F, Vergnani L, Manfredini R, Fersini C . Endocrine mechanisms of blood pressure rhythms. Ann N Y Acad Sci 1996; 783: 113–131.
Gangwisch JE . A review of evidence for the link between sleep duration and hypertension. Am J Hypertens 2014; 27: 1235–1242.
Mohsenin V . Obstructive sleep apnea and hypertension: a critical review. Curr Hypertens Rep 2014; 16: 482–484.
Sforza E, Saint Martin M, Barthelemy JC, Roche F . Association of self-reported sleep and hypertension in non-insomniac elderly subjects. J Clin Sleep Med 2014; 10: 965–971.
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A . Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009; 2: 491–497.
Fiorentini A, Valente R, Perciaccante A, Tubani L . Sleep’s quality disorders in patients with hypertension and type 2 diabetes mellitus. Int J Cardiol 2007; 114: E50–E52.
van den Berg JF, Tulen JH, NevenAK, Hofman A, Miedema HM, Witteman JC, Tiemeier H . Sleep duration and hypertension are not associated in the elderly. Hypertension 2007; 50: 585–589.
National Bureau of Statistics of China Series Books of the 2005 National Sample Survey of 1% of Population. China Statistics Press: Beijing. 2006.
Wang H, Zee P, Reid K, Chervin RD, Patwari PP, Wang B, Li Z, Tang G, Liu X, Yang J, Xu X, Wang X . Gender-specific association of sleep duration with blood pressure in rural Chinese adult. Sleep Med 2011; 12: 693–699.
Crundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C,, American Heart Association; National Heart, Lung and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American heard Association conference on scientific issues related to definition. Circulation 2004; 109: 433–438.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment on High Blood Pressure. Hypertension 2003; 42: 1206–1252.
Minh HV, Byass P, Chuc NT, Wall S . Gender differences in prevalence and socioeconomic determinants of hypertension: findings from the WHO STEPs survey in a rural community of Vietnam. J Hum Hypertens 2006; 20: 109–115.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213.
Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, Fang SC . Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res 2005; 14: 1943–1952.
Lo CM, Lee PH . Prevalence and impacts of poor sleep on quality of life and associated factors of good sleepers in a sample of older Chinese adults. Health Qual Life Outcomes 2012; 10: 72.
Li J, Yao YS, Dong Q, Dong YH, Liu JJ, Huang F . Characterization and factors associated with sleep quality among rural elderly in China. Arch Gerontol Geriatr 2013; 56: 237–243.
Wong WS, Fielding R . Prevalence of insomnia among Chinese adults in Hong Kong: a population-based study. J Sleep Res 2010; 20: 117–126.
Okubo N, Matsuzaka M, Takahashi I, Sawada K, Sato S, Akimoto N, Umeda T, Nakaji S . Relationship between self-reported sleep quality and metabolic syndrome in general population. BMC Public Health 2014; 14: 562.
Lu K, Ding R, Tang Q, Chen J, Wang L, Wang C, Wu S, Hu D . Association between self-reported global sleep status and prevalence of hypertension in Chinese adults: data from the Kailuan community. Int J Environ Res Public Health 2015; 12: 488–503.
National Institute on Alcohol Abuse and Alcoholism. Health risks and benefits of alcohol consumption. Alcohol Res Health 2000; 24: 5–11.
Huang Y, Mai W, Hua Y, Wu Y, Song Y, Qiu R, Dong Y, Kuang J . Poor sleep quality, stress status, and sympathetic nervous system activation in nondipping hypertension. Blood Press Monit 2011; 16: 117–123.
Erden I, Erden EC, Ozhan H, Basar C, Aydin M, Dumlu T, Alemdar R . Poor-quality sleep score is an independent predictor of nondipping hypertension. Blood Press Monit 2010; 15: 184–187.
Ben-Dov IZ, Bursztyn M . Nondipping in patients with hypertension. Hypertension 2009; 53: e35.
Yue JR, Wang H, Huang CQ, Dong BR . Association between sleep quality and arterial blood pressure among Chinese nonagenarians/centenarians. Med Sci Monit 2012; 18: PH36–PH42.
Batal O, Khatib OF, Bair N, Aboussouan LS, Minai OA . Sleep quality, depression, and quality of life in patients with pulmonary hypertension. Lung 2011; 189: 141–149.
Lee D, Heo SH, Yoon SS, Chang DI, Lee S, Rhee HY, Ku BD, Park KC . Sleep disturbances and predictive factors in caregivers of patients with mild cognitive impairment and dementia. J Clin Neurol 2014; 10: 304–313.
Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y . Hypertension and sleep: overview of a tight relationship. Sleep Med Rev 2014; 15: 1–11.
Guo X, Yu S, Li Z, Guo L, Zheng L, Yang H, Zou L, Hu W, Zhou Y, Zhu L, Zhang Y, Sun Y . Self-reported sleep duration is associated with reduced glomerular filtration rate among adults withhypertension: a population-based study from rural northeast China. J Sleep Res 2015; 24: 351–358.
Paciência I, Barros H, Araújo J, Ramos E . Association between sleep duration and blood pressure in adolescents. Hypertens Res 2013; 36: 747–752.
Meng L, Zheng Y, Hui R . The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res 2013; 36: 985–995.
Acknowledgements
We are very grateful to the participants from northeast China. We thank the anonymous reviewers for their very helpful comments. This research was supported by grants from the Liaoning Province Education Department Foundation (L2010672), the Liaoning Province Science and Technology Foundation (2013225049) and Guangdong Province Natural Science Foundation (2014A050503027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, RQ., Qian, Z., Trevathan, E. et al. Poor sleep quality associated with high risk of hypertension and elevated blood pressure in China: results from a large population-based study. Hypertens Res 39, 54–59 (2016). https://doi.org/10.1038/hr.2015.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.98
Keywords
This article is cited by
-
Sleep characteristic profiles and the correlation with spectrum of metabolic syndrome among older adult: a cross-sectional study
BMC Geriatrics (2022)
-
Interaction between trouble sleeping and depression on hypertension in the NHANES 2005–2018
BMC Public Health (2022)
-
Effect of antihypertensive medications on sleep status in hypertensive patients
Sleep and Biological Rhythms (2022)
-
Sex differences in the association between dinner–bedtime interval and abdominal obesity: a large-scale cross-sectional study
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Socioeconomic and lifestyle determinants of the prevalence of hypertension among elderly individuals in rural southwest China: a structural equation modelling approach
BMC Cardiovascular Disorders (2021)