Abstract
The (pro)renin receptor ((P)RR) is known to play an important role in the pathogenesis of vascular complications in diabetes mellitus and hypertension through its function in activating the local renin–angiotensin system. Recent studies have shown that the (P)RR is an accessory protein of the vacuolar H+-ATPase, suggesting a more fundamental and developmental function. In this study, smooth muscle cell-specific (P)RR/Atp6ap2 conditional knockout mice were generated. Smooth muscle cell-specific ablation of the (P)RR resulted in nonatherogenic sclerosis in the abdominal aorta. The deletion of the (P)RR did not affect ambulatory blood pressure levels. In cultured murine vascular smooth muscle cells (VSMCs), ablation of the (P)RR suppressed the expression of the Vo subunit c of the vacuolar H+-ATPase and impaired the cell recycling system, leading to autophagic cell death. In addition, loss of the (P)RR in VSMCs induced the expression of monocyte chemotactic protein-1 and interleukin-6 mRNAs. These results suggest that the (P)RR is essential for cell survival and downregulation of vascular inflammation in murine VSMCs through maintaining normal function of the vacuolar H+-ATPase.
Similar content being viewed by others
Introduction
The (pro)renin receptor ((P)RR) is reported to have a significant role in the pathogenesis of angiopathy developed in animal models of diabetes mellitus and hypertension.1, 2, 3, 4, 5, 6, 7 In human vascular smooth muscle cells (VSMCs), stimulation of the (P)RR with (pro)renin causes an angiotensin II-independent intracellular signal.8 In addition, overexpression of the human (P)RR in VSMCs of rats results in the elevation of blood pressure,9 and studies in Japanese and Caucasian populations have shown a significant association between (P)RR gene polymorphisms and blood pressure.10, 11 However, the physiological role of the (P)RR in VSMCs remains unclear. Ablation of the (P)RR gene in cardiomyocytes and renal glomerular epithelial cells showed that the (P)RR significantly contributes to the assembly and function of vacuolar H+-ATPase (V-ATPase), and thereby contributes to cell survival through maintaining V-ATPase dependent autophagy.12, 13, 14 As V-ATPase is implicated in a variety of basic cellular processes in different kinds of cells,15 we also examined the physiological roles of (P)RR in V-ATPase in VSMCs.
Methods
Generation of smooth muscle-specific Atp6ap2 conditional-knockout mice
The generation of Atp6ap2 floxed mice has been described in detail elsewhere.12 Briefly, in the present study, Atp6ap2 floxed mice were bred with mice expressing the Cre recombinase under the control of the myosin heavy chain (Myh11) promoter (The Jackson Laboratory, Bar Harbor, ME, USA). The resulting Atp6ap2lox/Y; Myh-11-Cre+/0 mice represent smooth muscle cell-specific Atp6ap2 conditional-knockout (cKO) mice. The control male mice were littermates that were heterozygous for either Atp6ap2 or Myh-11-Cre (Myh-11-Cre+/0; Atp6ap2+/Y), thereby excluding Cre- and flox-mediated toxicity as the basis for phenotypic disparity.
Measurement of arterial pressure
At 6 weeks of age, we implanted a telemetry transmitter probe (model TA11PA-C10, Date Sciences International, St. Paul, MN, USA) into mice under sodium pentobarbital anesthesia (5 mg kg−1 intraperitoneal) and the flexible tip of the probe was positioned immediately below the carotid arteries. The transmitter was sutured into the abdominal wall, and the incision was closed. Systolic blood pressure, diastolic blood pressure and heart rate were recorded in conscious, freely moving animals. Individual blood pressure, heart rate and activity waveforms were sampled every 10 min throughout the course of the study. Daily averages and s.d. values were then calculated with the Dataquest IV system (Data Sciences International) that consisted of the implanted radiofrequency transmitter and a receiver placed under each cage. The output was relayed from the receiver through a consolidation matrix to a personal computer. All animal experiments including radio telemetry transmitters were reviewed and approved by the Institutional Animal Care and Use Committee at Keio University School of Medicine.
Plasma renin activity and renal renin content
Immediately after killing, 1 ml blood specimen was collected from heart into a tube that contained EDTA-2Na (0.1 mg μl−1). After centrifugation for 15 min at 3000 r.p.m., the plasma was used to determine plasma renin activity with a commercially available RIA kit, according to the manufacturer’s instructions (TFB, Tokyo, Japan). For the measurement of renal renin content, a part of the removed kidney was weighed 50 mg, placed in 330 μl of buffer containing 2.6 mM EDTA, 1.6 mM dimercaprol, 3.4 mM 8-hydroxyquinoline sulfate, 0.2 mM PMSF and 5 mM ammonium acetate, homogenized with a chilled glass homogenizer and then centrifuged. Total renin content in the kidneys was determined as previously reported,16 using a commercially available RIA kit (TFB).
Histology
Samples of abdominal aortas were fixed overnight in 10% formalin at 4 °C, dehydrated with 70% ethanol, mounted in paraffin and sectioned (5 μm thickness). The aorta sections were stained by the Masson trichrome method for fibrosis and α-smooth muscle actin (α-SMA). Low and high-magnification fields were analyzed using BIOREVO (BZ-9000; Keyence, Osaka, Japan). The number of VSMCs was quantified by counting α-SMA-positive cells using a frame (2500 μm2) that was moved stepwise along the aorta. Results were then averaged.
Immunofluorescent staining of cultured VSMCs was performed 5 days after adenovirus transfection as described previously.8, 17 For the labeling of acidic organelles, cells were incubated with LysoTracker (Molecular Probes, Eugene, OR, USA) for 30 min, and then fixed with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4). The images were acquired using a confocal microscope (LSM700-ZEN; Carl Zeiss, Jena, Germany).
The tissues were also fixed in 2% paraformaldehyde, 2.5% glutaraldehyde and 0.1 M cacodylate buffer, prepared according to a standard protocol. Samples were prepared for transmission electron microscopy using the RMC MT6000 ultramicrotome (Tucson, AZ, USA), and visualized using the Hitachi H7500 electron microscope (Tokyo, Japan) and 2K × 2 K Gatan CCD camera (Tokyo, Japan).
Antibodies
Antibodies against V-ATPase subunits and RAB7 were as described previously.18, 19, 20, 21 Other primary antibodies used were monoclonal antibodies for LAMP2 (DSHB, Iowa City, IA, USA), ATP6AP2 polyclonal antibody (R&D Systems, Minneapolis, MN, USA), LC3 polyclonal antibody (a kind gift from Dr Komatsu, the Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), anti-α-SMA mouse monoclonal antibody (Dako, Glotnip, Denmark) and anti-β-actin mouse monoclonal antibody (Sigma, St Louis, MO, USA). Fluorescent dyes and enzyme-linked secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA, USA).
Gene expression
We extracted total RNA from a sample of the aorta from each animal and from VSMCs with an Rneasy Mini kit (Qiagen, Venlo, Netherlands). Real-time quantitative RT–PCR was performed using the TaqMan One-Step RT–PCR Master Mix Reagents kit and an ABI Prism 7700 HT Detection System (Applied Biosystems, Carlsbad, CA, USA). Primers and probes for (P)RR and GAPDH were used.12, 13 Other gene-specific primer and probe sets (TaqMan Gene Expression Assays) were as follows: forward 5′-CGGCTGGAGCATCCACGT-3′, reverse 5′-ATTGGGATCATCTTGCTGGTGAAT-3′ and probe 5′-FAM-TCAGCCAGATGCAGTTAACGCCCCAC-TAMRA-3′ for MCP-1; forward 5′-CCGGAGAGGAGACTTCACAGA-3′, reverse 5′-GTTGTTCATACAATCAGAATTGCCATT-3′ and probe 5′-FAM-ACCACTCCCAACAGACCTGTCTATACCACT-TAMRA-3′ for IL-6; forward 5′-TGCACTGGACCCTGGCTTTA-3′, reverse 5′-GGACTTCTGCTCTCCTTCTGTC-3′ and probe 5′-FAM-TGCTGTACCTCCACCATGCCAAGTGGT-TAMRA-3′ for VEGF.
Western blotting
Protein lysate preparation and immunoblotting procedures were performed as described previously.5, 8 Total lysates were applied at 10–20 μg per lane on 5–20% gradient polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene difluoride membranes with 0.2 μm pore size. Immunoblotting was performed using the aforementioned antibodies. The protein blots were developed with either an ECL detection kit (Thermo Scientific, Rockford, IL, USA) or chemiluminescence (Amersham Biosciences, Glattbrugg, Switzerland), and images were obtained using an image capture system (model LAS3000 luminoimager, Fujifilm, Tokyo, Japan).
Cell culture
Preparation of VSMCs from mice was performed as previously described.22 Thoracic aortae were removed from neonatal male Atp6ap2 floxed mice and digested in collagenase (1 mg ml−1, type II; Sigma) in Dulbecco’s modified Eagle’s medium (Sigma) for 30 min at 37 °C. After triturating and centrifuging twice, the cells were seeded in 10-cm cultured dishes and cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37 °C and 5% CO2. The culture medium was changed every second day. At 10–14 days after primary culture, the cells were trypsinized and seeded for the experiments to follow. The cells exhibited a ‘hill and valley’ growth pattern and were characterized by positive immunostaining with monoclonal antibodies against α-SMA. All experiments were performed using cells that were between 4 and 10 passages. The ablation of (P)RR/ATP6AP2 in cultured VSMCs was performed by the transfection of a recombinant adenovirus vector expressing a CRE mutant (50 multiplicity of infection) as previously described.23
Statistics
Data are presented as the mean ±s.d. The significance of differences between two mean values was evaluated using a two-tailed unpaired Student’s t-test. Multiple comparisons involving more than three groups were performed using analysis of variance. P<0.05 was considered significant.
Results
The cKO mice were born at the expected Mendelian frequency, and the (P)RR deletion was confirmed at genotype, protein and messenger RNA levels (Figures 1a–c). The average body weight of the cKO mice was significantly lower than that of wild-type (WT) mice after the mice were 6 weeks old. There was no difference in the amount of feed consumed by both groups. The cKO mice died at up to 20 weeks after birth (Figure 1d). At 6 weeks of age, there were no significant morphological anomalies; however, collapsed lungs were observed by 13 weeks in the cKO mice. Histological examination at 6 weeks showed damaged bronchial tubes and collapsed alveoli, although there were no significant abnormal findings in other smooth muscle-containing organs. There was no significant difference in ambulatory arterial pressure between the cKO mice and WT littermates by 16 weeks of age (Figure 1e). There was no significant difference in the renal renin content between WT (n=8) and cKO mice (n=4) in 6-week-old mice (5.6±0.5 vs. 5.5±0.4 ng per g kidney weight). Moreover, there was no significant difference in the plasma renin activity between WT (n=6) and cKO mice (n=3) in 6-week-old mice (22.7±11.8 vs. 24.7±17.6 ng of AngI ml h−1).
Genotyping of mouse genomic DNA using PCR from the tail of 6-week old mice, showing conditional deletion of floxed Atp6ap2 contingent on coinheritance of Myh11-Cre (a). Immunoblotting for Atp6ap2/(pro)renin receptor ((P)RR) protein in mouse abdominal aorta (b). Real-time PCR for Atp6ap2 mRNA from mouse abdominal aorta (c, n=3 *P<0.05 vs. wild-type (WT) control). Kaplan–Meier survival curve (d, n=8 in each). Ambulatory arterial blood pressure (e, n=4 in each).
Histological examination of the cKO mice revealed that they exhibited some vacuolated VSMCs at 13 weeks of age and that the 20-week-old cKO mice exhibited a decreased number of VSMCs and an increased number of fibroblasts without plaques in the abdominal aorta suggesting nonatherogenic sclerosis (Figure 2). Destructive elastic laminae were observed in the 20-week-old cKO mice. The number of VSMCs was similar between WT and cKO mice in 6-week-old mice (2910±320 vs. 3230±400 mm−2, n=4) but it was significantly decreased in 20 week-old cKO mice (6010±980 vs. 1180±280 mm−2, n=4, P<0.05 vs. WT mice). Although there was no apparent accumulation of inflammatory cells around the abdominal aorta, they were observed in smaller arteries such as the heart coronary arteries.
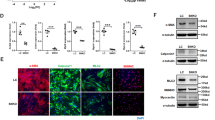
Electron microscopic examination of abdominal aortic VSMCs in 16-week-old cKO mice revealed the accumulation of numerous multivesicular vacuoles and enlarged autophagic vacuoles containing undigested cellular constituents (Figure 3). In addition, microtubule-associated protein 1 light chain 3 (LC3-II) expression was enhanced in VSMCs treated with adeno-Cre ((P)RR knockout VSMCs; Figure 4a). Similar results were seen in (P)RR knockout cardiomyocytes.12 Moreover, in ATP6AP2-depleted myocardium, increased expression of LC3-II and p62/SQSTM1 was observed, as well as the induction of genes responsive to amino acid starvation. These findings appeared to reflect defective autophagic protein degradation.12 It may be that impaired autophagic degradation also occurs in (P)RR knockout VSMCs. To investigate the mechanism of autophagic cell death, VSMCs were harvested from the thoracic aorta of male Atp6ap2 floxed mice, and infected with Cre-expressing adenovirus (adeno-Cre) to delete Atp6ap2/(P)RR. The transduction efficiency of adenovirus into VSMCs was analyzed by using green fluorescent protein-positive adenovirus. The ratio of green fluorescent protein-positive VSMCs to untreated VSMCs on the third day after infection was 99.4%, counted by fluorescence-activated cell sorting. We confirmed that adenovirus infection did not affect the expression of Atp6ap2 mRNA (date not shown). Western blot and real-time PCR analyses showed that the Atp6ap2/(P)RR protein was reduced by over 90% in a time-dependent manner after adeno-Cre treatment (Figures 4a and b). Western blot analyses revealed that the level of subunit c, a component of Vo sector V-ATPase, decreased in the Atp6ap2 floxed VSMCs treated with adeno-Cre. In contrast, the level of subunit E2, a component of the V1 sector V-ATPase, was unaffected. These results showed that in cultured VSMCs, the loss of ATP6AP2/(P)RR decreased the stability of membrane-bound Vo subunit of V-ATPase. In addition, LC3-II, which is expressed in autophagosomal isolation membranes, accumulated in the VSMCs treated with adeno-Cre (Figure 4a).
Immunoblotting for ATP6AP2/(pro)renin receptor ((P)RR) protein from vascular smooth muscle cells (VSMCs) derived from Atp6ap2 floxed mice treated with the Cre adenovirus infection (a). Real-time PCR for Atp6ap2 mRNA from VSMCs infected with Cre adenovirus (b, n=3 in each). Time-course analysis of vacuolar H+-ATPase (V-ATPase) subunit-c, subunit-E2 and LC3-II from VSMCs infected with Cre adenovirus (a). Immunofluorescence staining of RAB7, LAMP2 and LysoTracker in VSMCs treated with control adenovirus or Cre adenovirus (c). Scale bars: 10 μm.
Immunofluorescence analysis for VSMCs revealed that the Atp6ap2/(P)RR-depleted VSMCs had increased expressions for RAB7 and LAMP2, markers for late-endosome/lysosome as well as autophagosome-lysosome fusion, and that the loss of Atp6ap2/(P)RR impaired the VSMC acidic environment (Figure 4c). Thus, the genetic ablation of Atp6ap2 resulted in inhibiting assembly and function of V-ATPase, thereby compromising vesicular acidification in VSMCs.
We also assessed whether the ablation of Atp6ap2/(P)RR in VSMCs affects the production of chemokines and cytokines, such as monocyte chemotactic protein-1 (MCP-1), interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF). The MCP-1 and IL-6 mRNA levels increased 4.7-fold and 3.3-fold, respectively, compared with control VSMCs infected with only adenovirus. Control adenovirus infection did not change the MCP-1 and IL-6 mRNA levels significantly. However, there was no significant difference in the VEGF mRNA levels (Figure 5). To further investigate whether V-ATPase contributes to the elevated expression of MCP-1 and IL-6 mRNAs, we treated cultured VSMCs with the specific V-ATPase inhibitor, bafilomycin A1, at 100 nmol l−1 for 6 h. The VSMC MCP-1 and IL-6 mRNA levels increased 8.7-fold and 3.8-fold, respectively. These levels were similar to levels after ablation of Atp6ap2/(P)RR.
Levels of monocyte chemotactic protein-1 (MCP-1, a), interleukin-6 (IL-6, b) and vascular endothelial growth factor (VEGF, c) mRNAs (n=3 in each) in vascular smooth muscle cells (VSMCs) derived from Atp6ap2 floxed mice treated with control adenovirus, Cre adenovirus or bafilomycin A1. *P<0.05 vs. control. NS, not significant.
Discussion
In the abdominal aorta of the cKO mice, degeneration of VSMCs and proliferation of fibroblasts were observed. Electron microscopic examination of abdominal aortic smooth muscle cells from cKO mice revealed the accumulation of numerous multivesicular vacuoles and enlarged autophagic vacuoles containing undigested cellular constituents. This accumulation was caused by impairment of autophagic degradation, consistent with the previous studies using cardiomyocyte- and renal glomerular epithelial cell-specific cKO mice.12, 13, 14 Thus, (P)RR in VSMCs appears to be essential for autophagy and late-endosome/lysosome functions. The biosynthesis of the multisubunit V-ATPase complex requires the integration of V1 subunits, assembling in the cytosol, with VO subunits that are targeted to the vacuolar membrane in a concerted manner.24 Studies in yeast cells have shown that the loss of any single VO subunit affects the stability and assembly of the remaining VO subunits.24 There are two possible mechanisms responsible for the finding that the (P)RR knockout reduces expression of the Vo subunit c of V-ATPase. First, the Vo subunit mRNA levels could have been decreased. The (P)RR is an adaptor protein linking Wnt (co)receptors with the V-ATPase complex.25 Loss of (P)RR in the knockout may therefore suppress the transcription of the Vo subunit gene that may be dependent on Wnt signaling. Alternatively, the (P)RR deletion may affect the stability of the Vo subunit. Regardless, the ablation of (P)RR in mouse VSMCs resulted in decreased levels of a component of the Vo subunit of V-ATPase, thereby impairing its function. V-ATPase is located in the membranes of cytoplasmic organelles such as the Golgi apparatus, endosomes and lysosomes, and transports protons from the cytosol into the vesicles in an ATP-dependent manner.26, 27 The vesicular acidic environment, generated by V-ATPase, is known to be essential for normal membrane trafficking, membrane fusion and lysosomal protease functions.28 LysoTracker staining, which selectively stains acidic compartments, revealed impaired vesicular acidification in cultured (P)RR-null VSMCs. In addition, LC3-II, which is normally expressed in autophagosome membranes, was overexpressed in these cells, suggesting an impaired digestive process despite an enhanced phagocytosis. (P)RR-null VSMCs were also strongly positive for LAMP2 and RAB7, markers for autophagosome–lysosome fusion. Taken together, these results show that (P)RR is also essential for membrane trafficking, membrane fusion and autophagy through maintaining a V-ATPase-dependent acidic environment in VSMCs. Loss of these essential functions in the (P)RR knockout resulted in autophagic cell death in VSMCs.
There were no significant differences in the plasma renin activity and the renal renin content between WT and cKO mice. Although the overexpression of (P)RR in the smooth muscle cells was reported to increase plasma aldosterone concentrations through elevating local angiotensin II levels,9 it is unlikely that the deletion of (P)RR in the smooth muscle cells affects the local and circulating renin–angiotensin system. Thus, a gain of function in the (P)RR-related system results in the stimulation of the renin–angiotensin system, whereas a loss of function in the (P)RR-related system may be involved in the renin–angiotensin system-independent mechanisms.
In vitro deletion of (P)RR in VSMCs was accompanied by increased expression of IL-6 and MCP-1. The elevation of MCP-1 and IL-6 mRNA levels was also observed in VSMCs treated with the specific V-ATPase inhibitor, bafilomycin. The Vo domain of V-ATPase has been implicated in exocytosis that involves a fusion between the plasma membrane and vesicle membranes.29 It may be that deletion of (P)RR results in decreased secretion of MCP-1 and IL-6 through a dysfunction of exocytosis, and this might somehow stimulate intracellular production of MCP-1 and IL-6. Further studies are needed to determine how the (P)RR contributes to the regulation of these molecules.
In conclusion, as well as in cardiomyocyte and renal glomerular epithelial cells, (P)RR is essential for assembly and function of V-ATPase in VSMCs and plays an important role in the cell recycling system through V-ATPase-dependent mechanisms. In addition, the VSMC (P)RR may play an inhibitory role in the pathogenesis of nonatheromatous arterial fibrosis perhaps in part through downregulation of MCP-1 and IL-6.
References
Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M . Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 2006; 47: 894–900.
Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T . Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 2006; 17: 1950–1961.
Susic D, Zhou X, Frohlich ED, Lippton H, Knight M . Cardiovascular effects of prorenin blockade in genetically spontaneously hypertensive rats on normal and high-salt diet. Am J Physiol Heart Circ Physiol 2008; 295: H1117–H1121.
Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T . Inhibition of diabetic nephropathy by a decoy peptide corresponding to the ‘handle’ region for nonproteolytic activation of prorenin. J Clin Invest 2004; 114: 1128–1135.
Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H . Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J Am Soc Nephrol 2007; 18: 2054–2061.
Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H . Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens 2008; 2: 332–340.
Ichihara A, Sakoda M, Kurauchi-Mito A, Narita T, Kinouchi K, Murohashi-Bokuda K, Itoh H . Possible roles of human (pro)renin receptor suggested by recent clinical and experimental findings. Hypertens Res 2010; 33: 177–180.
Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H . Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res 2007; 30: 1139–1146.
Burckle C, Bader M . Prorenin and its ancient receptor. Hypertension 2006; 48: 549–551.
Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, Sugimoto K, Katsuya T, Ohkubo T, Hashimoto J, Rakugi H, Takahashi K, Imai Y . Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens 2009; 22: 294–299.
Ott C, Schneider MP, Delles C, Schlaich MP, Hilgers KF, Schmieder RE . Association of (pro)renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet Genomics 2011; 21: 347–349.
Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H . The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 2010; 107: 30–34.
Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada GH, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H . Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 2011; 22: 2203–2212.
Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfurst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN . Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 2011; 22: 2193–2202.
Nishi T, Forgac M . The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 2002; 3: 94–103.
Kobori H, Ichihara A, Suzuki H, Miyashita Y, Hayashi M, Saruta T . Thyroid hormone stimulates renin synthesis in rats without involving the sympathetic nervous system. Am J Physiol 1997; 272: E227–E232.
Sun-Wada GH, Imai-Senga Y, Yamamoto A, Murata Y, Hirata T, Wada Y, Futai M . A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J Biol Chem 2002; 277: 18098–18105.
Nakamura N, Yamamoto A, Wada Y, Futai M . Syntaxin 7 mediates endocytic trafficking to late endosomes. J Biol Chem 2000; 275: 6523–6529.
Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M . Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 2000; 275: 8760–8765.
Nakamura N, Sun-Wada GH, Yamamoto A, Wada Y, Futai M . Association of mouse sorting nexin 1 with early endosomes. J Biochem 2001; 130: 765–771.
Sun-Wada GH, Wada Y, Futai M . Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct 2003; 28: 455–463.
Akishita M, Ito M, Lehtonen JY, Daviet L, Dzau VJ, Horiuchi M . Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J Clin Invest 1999; 103: 63–71.
Tokunou T, Ichiki T, Takeda K, Funakoshi Y, Iino N, Takeshita A . cAMP response element-binding protein mediates thrombin-induced proliferation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2001; 21: 1764–1769.
Forgac M . Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 2007; 8: 917–929.
Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C . Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 2010; 327: 459–463.
Nelson N . Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci 1991; 12: 71–75.
Forgac M . Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev 1989; 69: 765–796.
Paroutis P, Touret N, Grinstein S . The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004; 19: 207–215.
Liegeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M . The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 2006; 173: 949–961.
Acknowledgements
We thank Drs Shu Takeda, Yoko Ogawa and Kenichi Matsushita for their kind cooperation and discussion, and Satoko Sunamura, Toshihiro Nagai and Katsuaki Dan for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kurauchi-Mito, A., Ichihara, A., Bokuda, K. et al. Significant roles of the (pro)renin receptor in integrity of vascular smooth muscle cells. Hypertens Res 37, 830–835 (2014). https://doi.org/10.1038/hr.2014.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.92
Keywords
This article is cited by
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
ATP6AP2 knockdown in cardiomyocyte deteriorates heart function via compromising autophagic flux and NLRP3 inflammasome activation
Cell Death Discovery (2022)
-
(Pro)renin receptor contributes to renal mitochondria dysfunction, apoptosis and fibrosis in diabetic mice
Scientific Reports (2019)
-
The (pro)renin receptor in health and disease
Nature Reviews Nephrology (2019)
-
Toll-like receptor 4 signaling has a critical role in Porphyromonas gingivalis-accelerated neointimal formation after arterial injury in mice
Hypertension Research (2016)